More Information
Submitted: March 31, 2025 | Approved: April 07, 2025 | Published: April 08, 2025
How to cite this article: Jha A. Toxic Components in Baby Care Products – A Comprehensive Review. J Forensic Sci Res. 2025; 9(1): 029-036. Available from:
https://dx.doi.org/10.29328/journal.jfsr.1001077
DOI: 10.29328/journal.jfsr.1001077
Copyright License: © 2025 Jha A. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Toxicants; Health risks; Developmental disorder
Toxic Components in Baby Care Products – A Comprehensive Review
Avinav Jha*
Student, MSc Forensic Science, Centre of Forensic Science, Department of Chemistry, Institute of Science, Banaras Hindu University, Varanasi, 221005, India
*Address for Correspondence: Avinav Jha, Student, MSc Forensic Science, Centre of Forensic Science, Department of Chemistry, Institute of Science, Banaras Hindu University, Varanasi, 221005, India, Email: [email protected]
Background: In addition to being used to keep babies clean and comfortable, baby care products may also include hazardous substances that are harmful to the baby’s health. To safeguard the health of new-borns, it is crucial to understand the potential toxins included in baby care products.
Objective: This paper focuses on the very bothering aspect of baby care products. The objective of this study is to identify and summarise the effect of toxicants present in baby care products including their source, exposure, toxicity, and adverse effects on infants.
Methods: Utilizing several internet databases including various open source, including PubMed, Scopus, and research gate, a thorough literature search was carried out. The review covered articles that were written in English and published in last fifteen years. Studies reporting on the sources, effects, and potential exposure pathways of toxicants found in infant care products have been included.
Result: The study deals with a list of harmful toxicants like phthalates, asbestos, parabens, heavy metals, sodium laurel sulphates, etc., and their sources and modes of exposure. Exposure to toxicants such as phthalates, asbestos, parabens, heavy metals, and sodium laurel sulphates can lead to cancer, developmental disorders, and endocrine disruption.
Conclusion: It can be concluded that baby care products are having adverse effects on infants, on their skin or health, or in other ways. To avoid the same, the root cause of it should be avoided, which is the inclusion of toxicant chemicals in such baby care products. Parents and caretakers should be aware of the dangers of toxicant chemicals in baby care products and use non-toxic products to protect their babies' health, while manufacturers should use safer components. Government and authorized agencies should enforce restrictions.
The baby care industry includes any product that satisfies the demand for looking after an infant, typically between the age group of 0 to 4 years. India is the most populous nation in the world, right behind is China, with 1.28 billion people, 27 million infants born per year, and a fertility rate of 2. 2 India accounts for 20%, or roughly 127 million, of the world's 0–4-year-old population. According to estimates, there will be 27 million live pregnancies in the nation this year, or 20% of all live births worldwide. In terms of revenue, the market of baby care products is predicted to increase by an average annual rate of over 17% from 2014 to 2019 to hit over $31 billion from $14 billion, making India a lucrative market. The size of the global market for infant products was forecast to be USD 214.13 billion in 2021, and from 2022 to 2030, it is anticipated to rise at a compound yearly growth rate of 5.7% [1]. Skin is regarded primary layer of protection. An infant's skin is especially prone to skin breakdown, allergies, extravasation, and infections because of its delicate nature. Consequently, it is essential to take action to preserve skin health [2] Skincare products are used to keep the skin healthy, and they are becoming increasingly popular both in the Indian market and internationally. There are many brands dealing in baby care products worldwide including India [3]. As per some paediatric publications, it is recommended to use pH-neutral cleansers while bathing babies. Skin-softening products used on babies should be free from preservatives, dyes, or fragrances. It is important to keep the nappy area clean and moisturized [4,5].
They have a vast selection of baby care products, specially designed for babies, which includes moisturizers, soap, shampoo, hair oil, diaper, fruit silicone nibbler, lotion, cream, wet tissue, kajal, diaper rash cream, body wash, etc. Various toxic chemicals are present in baby care products, a potentially harmful cause of toxicity for an infant's body is the existence of heavy metals. Several heavy metals have been implicated in human poisoning, including lead, mercury, arsenic, and cadmium. These heavy metals cause genotoxicity and cancer in humans. Heavy metal toxicity can result from industrial contact, air or water pollution, eating contaminated food or taking contaminated medicine, using poorly coated food containers, or consuming paint that contains lead [6]. Water-based products often contain formaldehyde to inhibit the growth of microorganisms. Some products use formaldehyde-releasing preservatives (FRPs), such as dimethylol urea, DMDM hydantoin, polyoxymethylene urea, sodium hydroxymethylglycinate, and dialimidinyl urea, in place of formaldehyde. Infants may therefore be exposed to formaldehyde [7]. Formaldehyde is frequently associated with gene damage, mutations, and developmental issues and is a known cytotoxin and likely human carcinogen [8-10]. Several of the most popular kinds of baby care items are given in Table 1.
| Table 1: Baby care products and their uses. | ||
| S no. | Baby care products | Uses |
| 1. | Diapers | To keep baby dry and comfortable |
| 2. | Wipes | For cleaning body parts |
| 3. | Lotions & creams | To moisturize and protect baby skin from dryness and irritation |
| 4. | Powder | To absorb moisture and prevent diaper rash |
| 5. | Kajal | To enhance eye appearance and protect them from the sun. |
| 6. | Shampoos and washes | used to cleanse a baby's delicate skin and hair |
| 7. | Baby oil | Used to hydrate and safeguard delicate skin |
| 8. | Teething product | To soothe baby’s gum during the teething process |
Diaper
A diaper is a piece of towelling or an absorbent material that allows the baby to pee or defecate without using the toilet. It absorbs waste and helps to prevent contamination of outer clothing or the environment. If diapers get wet or dirty, it should be changed so that it doesn’t irritate the baby, usually by someone else, such as a parent or caretaker [11]. Innovations in diapers give parents more choices to consider using them according to their requirements and comfort. Diapers are made of several layers of material with different properties. Diapers contains superabsorbent materials that absorb and retain urine, keeping the skin of baby dry and clean [12]. A baby's skin is particularly sensitive to irritation, especially in the diaper area, where it comes into close contact with urine and faeces [13]. There are different types of diapers. They are traditional diapers, conventional diapers, and SA diapers [11]. Reusable diapers were the only diapers available until the 1960s when traditional disposable diapers were introduced. Table 2 shows the analytical techniques used to identify different xenobiotics in baby diapers.
| Table 2: Analytical techniques used to identify different xenobiotics in baby diapers. | ||||
| Group of xenobiotics | Impact of xenobiotics on baby’s health | Analysed part of diapers | Analytical techniques | References |
| Phthalates | Cause an allergic skin reaction and act as endocrine disruptors. | Top sheets | GC-MS, GC-FID |
[14-16] |
| Total residual acrylic acid | Adverse reaction on skin, eye, and throat | Superabsorbent polymer | GC-TCD | [17] |
| Monoaromatic hydrocarbons | Irritation after prolonged and repeated contact with skin. Redness and blister can be seen. Endocrine disruption is possible. | Complete diaper | GC-MS | [16] |
| Oragnotions | Have a variety of toxicity including immunotoxicity and other effects related to nervous system. | Diaper cover, top sheet | GC-FPD, ED-XRF |
[18,19] |
| Formaldehyde | It links to cancer, skin allergies, and irritation. | Lining and waterproof layer. | HPLC-UV | [20] |
| Benzothiazoles | Probably carcinogenic to humans. | Complete diaper | HPLC-ESI-MS/MS | [21] |
| Biocides, phenolic compounds, parabens, caprolactam and pentadecafluorooctanoi c acid (PFOA) | Estrogenic effects and skin irritation | Top sheet | HPLC-ESI-MS/MS | [22] |
| Bio phenols, benzophenones, and bisphenol A diglycidyl ethers. | Show the impact on brain and prostate gland and children’s behaviour | Complete diaper | HPLC-ESI-MS/MS | [21] |
| PCDDs | Cause skin disorders, liver problems, impairment of the immune system, effects on the developing nervous system, and certain types of cancers. | Cotton(textile) part of diapers | HRGC-MS | [23] |
| PAHs | Mutagenic and carcinogenic effects, endocrine distribution | Parts that are in contact with the skin of the baby. | GC-MS | [22] |
| Pesticide | May cause an allergic skin reaction and cancer. | Complete diapers without absorbents. | UPLC-MS/MS | [22] |
Wipes
It’s a type of baby care product used for various purposes like cleaning the private parts, hands, or faces of babies. As babies’ skin is sensitive, these wipes must be made up of components that would be gentle for the baby’s skin and don’t cause them irritation or any kind of harm. The baby wipes have three basic parts i.e., base sheet, formulation, and package. The base sheet is the fabric that is used to produce the wipes. The formation includes substances that moisten the wipe and fulfil the purpose of wipes [24].
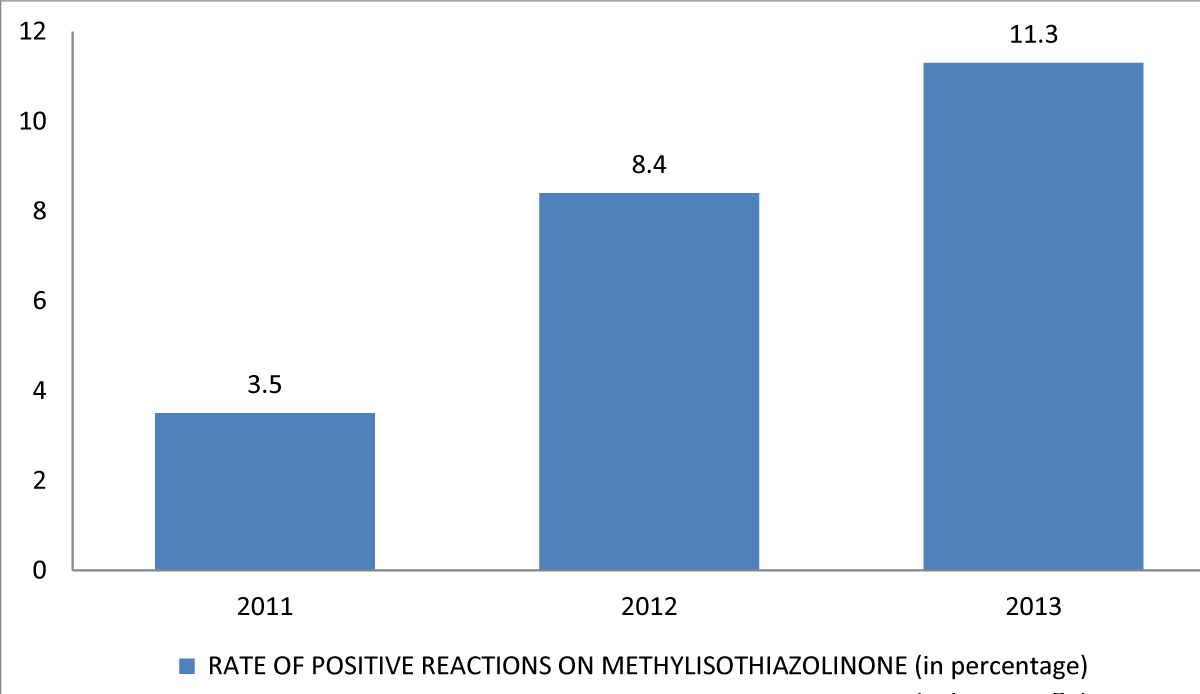
According to Kuller, et al. Baby wipes were the most often used product in the United States in 2014, followed by baby oil, lotion, and powder [25]. According to a study done by Cahill, et al. it was observed in Australia that several common brands of baby wipes contain a chemical named methylisothiazolinone due to which the allergy has increased greatly in patients [26]. In comparison to rates of 3.5% (15/428) in 2011 and 8.4% (38/454) in 2012, and the rate of positive reactions on methylisothiazolinone patch testing through November 2013 was 11.3% (40 patients of a total of 353 had meaningful reactions) Figure 1.
Figure 1: Positive reactions on methylisothiazolinone patch testing.
| Table 2: Analytical techniques used to identify different xenobiotics in baby diapers. | ||||
| Group of xenobiotics | Impact of xenobiotics on baby’s health | Analysed part of diapers | Analytical techniques | References |
| Phthalates | Cause an allergic skin reaction and act as endocrine disruptors. | Top sheets | GC-MS, GC-FID |
[14-16] |
| Total residual acrylic acid | Adverse reaction on skin, eye, and throat | Superabsorbent polymer | GC-TCD | [17] |
| Monoaromatic hydrocarbons | Irritation after prolonged and repeated contact with skin. Redness and blister can be seen. Endocrine disruption is possible. | Complete diaper | GC-MS | [16] |
| Oragnotions | Have a variety of toxicity including immunotoxicity and other effects related to nervous system. | Diaper cover, top sheet | GC-FPD, ED-XRF |
[18,19] |
| Formaldehyde | It links to cancer, skin allergies, and irritation. | Lining and waterproof layer. | HPLC-UV | [20] |
| Benzothiazoles | Probably carcinogenic to humans. | Complete diaper | HPLC-ESI-MS/MS | [21] |
| Biocides, phenolic compounds, parabens, caprolactam and pentadecafluorooctanoi c acid (PFOA) | Estrogenic effects and skin irritation | Top sheet | HPLC-ESI-MS/MS | [22] |
| Bio phenols, benzophenones, and bisphenol A diglycidyl ethers. | Show the impact on brain and prostate gland and children’s behaviour | Complete diaper | HPLC-ESI-MS/MS | [21] |
| PCDDs | Cause skin disorders, liver problems, impairment of the immune system, effects on the developing nervous system, and certain types of cancers. | Cotton(textile) part of diapers | HRGC-MS | [23] |
| PAHs | Mutagenic and carcinogenic effects, endocrine distribution | Parts that are in contact with the skin of the baby. | GC-MS | [22] |
| Pesticide | May cause an allergic skin reaction and cancer. | Complete diapers without absorbents. | UPLC-MS/MS | [22] |
The patient population's most typical source of allergic contact dermatitis during that time is methylisothiazolinone. Celeiro, et al. 2015 performed an experimental analysis of toxicants present in wipes of both national and international brands [27]. These were obtained from local sources and got preserved at normal temperatures. The extraction was taken by the pressurised liquid process, followed by gas chromatography mass spectrometry to detect and analyse the toxicants. A series of toxicants including fragrance allergens, preservatives, muska and phthalates were found to be present in the baby wipes. It was detected that a group of antimicrobials called parabens are used for the preservation of infant wipes from bacterial and mould growth.
In recent studies carried out, humans exposed to the parabens have been found to be privy to potential disrupting effects of endocrine and hormonal systems. Parabens are absorbed through dermal absorption, accounting for 66% of the exposure [28].
Baby lotion
Baby lotion is a moisturizing lotion that is specially made for infants and young children. It is made with the purpose of providing hydration and nourishment to the skin while being gentle and non-irritating. Baby lotion generally has a light & smooth texture that is easily absorbed into the skin, leaving it soft and flexible. Baby lotions are formulated to provide gentle and effective moisturization and protection to the baby's delicate skin. They contain many ingredients like water, glycerine, petrolatum, mineral oil, Isopropyl Palmitate, Glyceryl Stearate, Cetyl Alcohol, Stearic Acid, Cetearyl Alcohol, Oleic Acid, Benzyl Alcohol, Magnesium Aluminium Silicate, Methylparaben, Propylparaben, Sodium Hydroxide, Pentaerythrityl Tetra-di-t-butyl, and various emollients such as shea butter and oils like sunflower or coconut oil. Water is the main component that gives hydration to the skin. Glycerine is a humectant that attracts moisture from the environment. Petrolatum and mineral oil are occlusive agents that form a protective barrier on the skin & help on preventing moisture loss. It is helpful in healing dry or irritated skin. Emollients help to soften and smooth the skin, providing long-lasting hydration and protection. The use of methyl and propylparaben in baby lotion has been linked to the disruption of the endocrine glands on continuous exposure. These components have also been found to cause reproductive toxicity [29]. Methyl Paraben has been found to decrease the proliferating ability of keratinocytes and change cell morphology, as well as influence the aging and differentiation of keratinocytes [30]. Cetyl alcohol, when impure, has been found to cause allergic contact dermatitis, characterized by long-lasting friction, pressure, and sweating [31]. Stearic Acid and lignoceric acid have been found to exhibit radioactivity when in contact with skin fibroblast homogenates, which can be converted into water-soluble products [32]. Other components commonly used in baby lotion, such as mineral oil, benzyl alcohol, and propylene glycol, have been found to exhibit similar toxicity to that found in baby oil and baby wash. It is important to be aware of these harmful components and one should consider using alternative products that do not contain them. Major toxicants found in baby lotion and their effects are summarised in Table 3.
| Table 3:Toxicants in Baby Lotions and their effects. | |||
| S no. | Toxicants | Effects of toxicants | References |
| 1 | Methyl Paraben | Decrease the proliferating ability of keratinocytes, change cell morphology | [30] |
| 2 | Cetyl alcohol | Cause allergic contact dermatitis | [31] |
| 3 | Stearic Acid and lignoceric acid | Exhibit radioactivity when in contact with skin fibroblast homogenates | [32] |
Finely powdered magnesium silicate, together with possible traces of zinc and magnesium stearate, makes up talcum powder. In new-borns who are fat and prone to chafing and irritation at skin creases, talcum powder may play a little function as a drying agent. Talcum powder's non-absorbable nature might cause skin irritation if proper hygiene is not there [31]. In addition to other toxicants, asbestos has also been detected in baby care powder, but most people still prefer to use it [33]. Table 4 summarizes the ingredients present in baby care powder and their uses:
| Table 4: Harmful ingredients in powder and their amount. | |||
| S.No. | Ingredients | Formula (amount) | Uses |
| 1 | Lithium stearate | 2.50 | Act as water repellent |
| 2 | Stabilizer Kaolin | 5.0 | Act as an adsorbent |
| 3 | Talc | 90.25 | Act as slipping agent |
| 4 | Zinc oxide | 2.0 | Act as an opacifying agent |
| 5 | Perfume | 0.25 | Gives pleasant odour |
| 6 | Corn Starch | 99.70 | Act as adsorbent |
| 7 | Hyamine 10-X | 0.05 | Act as germicide |
Magon in 2014 conducted a study on the infant care practice of applying talcum powder in South India, because of ill health & death of infants, followed by accidental inhalation of baby powder [34]. The study was carried out at the Sri Lakshmi Narayana Institute of Medical Sciences and the study found that mothers used talcum powder on their infants both to soothe sweating and to make the infant smell better. Only 1% of people knew that talcum powder could harm a child. And 3 babies suddenly had an attack of cyanosis and violent cough soon after the use of talcum powder and were hospitalized for 5 days. All these children were found playing with talcum powder. Talc present in powder is insoluble in water, causing drying of the mucous membranes of the tracheobronchial tree, when inhaled, and can also cause mechanical obstruction of the small airways Oedema, and inflammation of the bronchial epithelium, leading to a pattern typical of acute lung injury. Talcosis can be caused by chronic inhalation of talc. And because it is a naturally occurring mined product, often contaminated with crystalline silica and asbestos. During processing, most of the asbestos from talc is removed, but traces may still be present and cause harm to infants and babies. The use of talc on wounds can result in the development of scabs, a risk of infection, and foreign body granulomas in the dermis [35]. Twenty consumer products, including body powders, baby powders, face talcums, and medicinal talc, were examined by [36]. 10 out of the 20 goods tested for tremolite and anthophyllite, primarily asbestiform, were found to contain these materials [36]. High inhalation exposures are likely to be the cause of infant deaths from talc asphyxiation during diapering [37]. When applied to the skin regularly, talcum powder contains recognizable asbestos fibres that might be discharged into the air and ingested. Talc inhalation has been linked to granulomatous nodules on the lungs known as talkies. Moreover, talc exposure has been linked to the emergence of mesothelioma, ovarian cancer, and gynaecological malignancies [38]. Talc can also cause eye irritation and lung damage if breathed. It is a component in diaper rash remedies and baby powders [39]. And because it is a naturally occurring mined product, often contaminated with crystalline silica and asbestos. During processing, most of the asbestos from talc is removed, but traces may still be present and cause harm to infants and babies. Karwoski, et al. in 2017 used graphite furnace atomic absorption spectroscopy for the analysis of Pb-blood levels in hospitalized infants on which diaper powder was used [40]. The study conducted in respect to blood lead level shows a range of 13-18 mcg/dL with the exposure, which decreased to 8 mcg/dL weeks after discontinuing use of diaper powder. The powder so used shows 62% lead by weight (PbO), 1000 ppm arsenic, 110 ppm bismuth, 31 ppm thallium by composition Figure 2.
Figure 2: Blood lead level before and after discontinuing of diaper powder.
Kajal
The term "kajal" denotes an ultra-fine powder made of many ingredients, such as herbs, pearls, gemstones, as well as other materials, as explained by the Unani, Ayurveda, and Greko-Arabica medical traditions for the eye [41], used to enhance the appearance of a baby's eyes and also makes it dark, but due to possible health risks, it is not advised to use upon infants.
Some kajal products may contain toxic chemicals (Table 5) such as lead, which can cause harm to infants if ingested or absorbed through the skin or eyes (US FDA: food and drug administration). According to studies, "Kajal" is made of magnetite (Fe3O4), zincite, amorphous carbon, galena (PbS), and minium (Pb3O4)(ZnO). Prolonged use of it can cause excessive storage of lead in the body, which may affect the brain and bone marrow and cause. Seizures and anaemia [42].
| :Toxicants present in kajal. | |||
| Sno. | Toxic Substance | Functions | Reference |
| 1. | Lead | Highly toxic heavy metal can cause developmental delays, cognitive impairment, and behavioural problems in infants and children. | [43] |
| 2. | Antimony | A heavy metal that Can cause harm to the eyes and skin | [44] |
| 3. | Aluminium | Generally, it does not cause harm but in the salt form, it can cause eye irritation and conjunctivitis, dermatoses, and eczema. | [45] |
| 4. | Camphor | Cause eye and skin irritation | [45] |
| 5. | Menthol | Can cause cold, & a burning sensation & excessive amount can cause dizziness, hallucination, convulsion & comma | [45] |
Al Ashban, et al. in 2004 performed a study on Kajal samples manufactured and sold in Saudi Arabia. A total of 107 samples of kajal, both branded and unbranded, were collected from various parts of Saudi Arabia and tested for lead [45]. The amounts of aluminium and arsenic were also detected. Some kajal products had lead amounts as high as 53%, and some samples had camphor and menthol in them. Regular kajal users' blood tests showed elevated lead concentrations and comparatively low haemoglobin levels. Scientists believe that using lead-based kajal could poison people, especially young children. Tiffany-Castigilioni, et al. in 2012 reviewed 26 studies published between 1968 and 2012 to support their finding that Pb based kajal is linked to higher PbB (blood lead) levels in women and children who use kajal [43]. Al Saleh, et al. conducted a study in Saudi Arabia in the year 1996, the author concluded that kajal was the main cause of elevated PbB in infants [46]. In another case study of a US infant who was 11 months old and was published in 1999, the author noted that the child's initial PbB level was 43 g/dL. The child's kajal treatment history dated back five months, and no other clear sources of Pb exposure were found. After stopping kajal for 8 weeks, the child's PbB was 23 g/dL. Kajal was determined to be the primary cause of lead poisoning.
Shampoo
Baby care shampoo is basically a type of shampoo specifically formulated for infants and young children, taking into consideration their soft and sensitive skin. Many baby care shampoos have been found to use toxic chemicals to be able to be tear-free, and not hurting or discomforting the eyes of the infants. Therefore, it becomes critical for the users to be more aware and vigilant to the ingredients used in such products. There are some toxic chemicals present in baby care shampoo they are discussed below. Phthalates are used in the production of dermally applied infant care products and are present in infant shampoo. Sathyanarayana, et al. found that phthalate exposure through ingestion, dermal transfer, inhalation, and various other routes [47]. Other than shampoo, phthalates are present in plastic products such as children’s toys, lotions, and other infant care products. Looking deeply into the constituents of shampoos, bath products and skin cleansers which create high level of foam and remove oil and dirt. Evaluation of ammonium and sodium laureth sulphate shows that the laureth sulphate salts can cause eye, skin, and respiratory irritation if present in more concentration [48]. The fragrances used in most baby care shampoos can contain a worrisome amount of different chemicals, causing allergic reactions and irritations on delicate baby skin.
Formaldehyde is a preservative that is utilized for the extended-term storage of products. It releases bronopol, Diazolidinyl urea, quaternium-15, and imidazolidinyl [49]. It has been known through the report of National Toxicology Program on carcinogens, that classified formaldehyde are human carcinogens.
The twenty personal care products to be tested under high performance liquid chromatography mass spectrometry (HPLC-MS), found five of them containing measurable levels of formaldehyde, concentrations ranging from 23 to 1500 ng/mg. The products under inspection included range of scented baby shampoos, baby washes and others. Of the five products, two contained formaldehyde concentrations exceeding 500 ng/mg (0.05%), which is the legal threshold for products marketed toward children in Minnesota. Notably, one of the products that exceeded this limit was the scented baby shampoo, which contained 580 ng/mg of formaldehyde [50]. According to study conducted by Groot, et al. two shampoo samples were found to contain detectable levels of formaldehyde [51]. One of the samples was marketed as a tear-free baby wash/shampoo and had a formaldehyde concentration of 23 ng/mg, which is relatively low and not expected to trigger an allergic response, even in individuals with high sensitivity. However, the scented baby shampoo contained a notably high concentration of formaldehyde at 580 ng/mg, and allergic reactions are frequently reported in individuals with formaldehyde sensitivity at this level. Moreover, there are additional concerns regarding this scented baby shampoo. The product is not labelled as containing formaldehyde or a formaldehyde releaser, and it is specifically marketed for use on infants, which raises further issues.
Baby wash
Baby wash is a sort of cleanser or purifying item that is explicitly formed for use on babies and small kids. It is made to cleanse effectively without stripping the skin's natural oils and to be gentle and non-irritating to babies' delicate skin. The components of baby wash can vary from brand to brand and product to product, but some common ones that are mostly used are mentioned below:
Water: Most baby washes are based on water, which is the main ingredient.Surfactants: Compounds like these remove oil and dirt from the skin and aid in cleansing. Surfactants like sodium lauryl sulphate, sodium laurel sulphate, and cocamidopropyl betaine are used in the baby wash.
Emollients: These are saturating fixings that assist to alleviate and safeguard the skin. Glycerine, petrolatum, and coconut oil are a few examples of emollients found in baby shampoo.
Humectants: These are fixings that assist to draw in and hold dampness in the skin. Propylene glycol and hyaluronic acid are two examples of humectants that are utilized in the baby wash.
Fragrance: Essential oils or fragrances may be added to some baby washes to give them a pleasant scent.
Preservatives: Preservatives like phenoxyethanol and benzyl alcohol is used in baby washes to stop the growth of bacteria and other microorganisms in the product (Table 6).
| :Toxicants present in kajal. | |||
| Sno. | Toxic Substance | Functions | Reference |
| 1. | Lead | Highly toxic heavy metal can cause developmental delays, cognitive impairment, and behavioural problems in infants and children. | [43] |
| 2. | Antimony | A heavy metal that Can cause harm to the eyes and skin | [44] |
| 3. | Aluminium | Generally, it does not cause harm but in the salt form, it can cause eye irritation and conjunctivitis, dermatoses, and eczema. | [45] |
| 4. | Camphor | Cause eye and skin irritation | [45] |
| 5. | Menthol | Can cause cold, & a burning sensation & excessive amount can cause dizziness, hallucination, convulsion & comma | [45] |
Baby oil
Baby oil is a mineral-based lubricant that is frequently utilized to hydrate and safeguard delicate skin. It is a clear, odourless liquid that is frequently advertised for use on infants but can be used on people of any age. Mineral oil, a clear, odourless, and light oil made from petroleum, is the main component of baby oil. Fragrance, vitamin E, aloe vera, and sometimes a small number of preservatives are other common ingredients found in baby oil. Mineral oils have been used in cosmetic and topical drug preparations for most of this century. They are excellent emollients and moisturizers in addition to serving as a lipophilic carrier for active ingredients. Because it forms a barrier that helps to prevent the loss of moisture, baby oil is typically used to moisturize and protect the skin. Because it can help to calm and relax the skin, it is also sometimes used as a massage oil. Baby oil, on the other hand, should not be inhaled or applied to the eyes or any other mucous membranes. Mineral Oil, USP, or Light Mineral Oil, NF is used in the formulations of baby oils, bath oils, creams and lotions, lipsticks and lip gloss, sunscreen, hair products, and makeup bases and removers in concentrations ranging from less than 1 to approximately 99 percent. Clearly, white mineral oils are used in a variety of ways that affect the appearance and performance of a wide range of food, pharmaceutical, and non-pharmaceutical products. In addition, it is likely that most people have been exposed to mineral hydrocarbons on an acute or chronic basis due to the widespread distribution and variety of products containing white mineral oils. Pharmaceutical mineral white oil, for instance, is utilized as a base for baby oils and as a laxative. Although mineral oil has many uses, prolonged or repeated exposure can cause skin irritation, rashes, and other skin problems, and inhaled mineral oil can cause inflammation and lung damage. Exogenous lipid pneumonia has been reported in humans following inhalation or accidental aspiration of mineral oil products [55,56]. If ingested in large quantities, paraffin liquidum can cause gastrointestinal problems such as nausea, vomiting, and diarrhoea. If inhaled It can also damage the lungs, so it becomes important to use baby oil and other mineral oil-based products with caution, avoiding inhalation and contact with eyes or mucous membranes [57]. In general, baby oil is an effective moisturizer and skin protectant made primarily using mineral oil. Although mineral oil has many uses and is widely used in these categories of products, it can cause skin irritation and other skin problems with prolonged or repeated exposure. Inhaling mineral oil can also lead to lung damage, and ingestion in large quantities can cause gastrointestinal issues. Therefore, it becomes important to use mineral oil-based products with caution and according to the product manufacturer's instructions.We can fairly infer from these discussions that our understanding of the safety risks posed by chemicals in baby care products has gradually increased. When chemicals like phthalates, parabens, and formaldehyde-releasing preservatives are used in infant care products with newborns and young children as the intended users, this can result in serious health issues like skin irritation, lung issues, hormonal disruption, and in some extreme cases, cancer. Moreover, there have been questions over the regulatory measures of baby care products. Chemicals are both inadequately regulated when being incorporated into baby care products as well as some chemicals are banned to use by some countries but are still being used in many places leading to a lack of worldwide consumers over the standard of safety and use/ misuse of chemicals in baby care products. All nations need to come together on the list of chemicals that can and cannot be used along with the ratio and percentage allowed as standards. This concern extends to organic products too rather than only chemical-based products. Labelling the products as organic or natural does not guarantee the safety of infant’s skin and body. Even these products can include hazardous ingredients in both quality and quantity since there is no standard for the regulation of these products. So, baby care products should be labelled with more transparency in the components used and the ratio of the ingredients rather than being ambiguous so that consumers have the ease of understanding and evaluating the safety of these products based on the labels and information provided. In conclusion, society need to collectively come forward to share the responsibility of trying to curb the use of hazardous and dangerous substances in baby products. This issue needs to be taken more seriously because it concerns the safety of infants. Government should release safety guidelines and regulatory standards and make sure that these standards are followed. While we as consumers need to choose products with minimal hazardous ingredients or rather prefer organic products to decrease the exposure to chemicals in infants and new-borns.
- Lekha SA. Consumer behaviour on baby care products in Madurai district young mothers perspective. 2019. Available from: https://www.vhnsnc.edu.in/img/Invitation/2020-2021/bba/Ph.D_Viva_Voce_BBA_250920.pdf
- Brod BA, Treat JR, Rothe MJ, Jacob SE. Allergic contact dermatitis: Kids are not just little people. Clin Dermatol. 2015 Nov 1;33(6):605–12. Available from: https://doi.org/10.1016/j.clindermatol.2015.09.003
- Nepalia A, Singh A, Mathur N, Pareek S. Toxicity assessment of popular baby skin care products from Indian market using microbial bioassays and chemical methods. Int J Environ Sci Technol. 2018;15(11):2317–24. Available from: https://link.springer.com/article/10.1007/s13762-017-1556-z
- Visscher M, Narendran V. Neonatal infant skin: Development, structure and function. Newborn Infant Nurs Rev. 2014;14(4):135–41. Available from: https://doi.org/10.1053/j.nainr.2014.10.004
- Kuller JMM. Update on newborn bathing. Newborn Infant Nurs Rev. 2014;14(4):166–70. Available from: https://doi.org/10.1053/j.nainr.2014.10.006
- Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. EXS. 2012;101:133–64. Available from: https://link.springer.com/chapter/10.1007/978-3-7643-8340-4_6
- Lv C, Hou J, Xie W, Cheng H. Investigation on formaldehyde release from preservatives in cosmetics. Int J Cosmet Sci. 2015;37(5):474–8. Available from: https://doi.org/10.1111/ics.12212
- Lovschall H, Eiskjaer M, Arenholt-Bindslev D. Formaldehyde cytotoxicity in three human cell types assessed in three different assays. Available from: http://www.elsevier.com/locate/toxinvit
- Ke YJ, Qin XD, Zhang YC, Li H, Li R, Yuan JL, et al. In vitro study on cytotoxicity and intracellular formaldehyde concentration changes after exposure to formaldehyde and its derivatives. Hum Exp Toxicol. 2014;33(8):822–30. Available from: https://doi.org/10.1177/0960327113510538
- Yoshida I, Ibuki Y. Formaldehyde-induced histone H3 phosphorylation via JNK and the expression of proto-oncogenes. Mutat Res. 2014 Dec 1;770:9–18. Available from: https://doi.org/10.1016/j.mrfmmm.2014.09.003
- Prasad HRY, Srivastava P, Verma KK. Diapers and skin care: Merits and demerits. Indian J Pediatr. 2004;71:907–8. Available from: https://doi.org/10.1007/bf02830834
- O’Connor RJ, Sanchez V, Wang Y, Gibb R, Nofziger DL, Bailey M, et al. Evaluation of the impact of 2 disposable diapers in the “Natural” diaper category on diapered skin condition. Clin Pediatr (Phila). 2019 Jun 1;58(7):806–15. Available from: https://doi.org/10.1177/0009922819841136
- Yuan C, Takagi R, Yao XQ, Xu YF, Ishida K, Toyoshima H. Comparison of the effectiveness of new material diapers versus standard diapers for the prevention of diaper rash in Chinese babies: A double-blinded, randomized, controlled, cross-over study. Biomed Res Int. 2018;2018:5874184. Available from: https://doi.org/10.1155/2018/5874184
- Razavi N, Es’haghi Z. Employ of magnetic polyaniline coated chitosan nanocomposite for extraction and determination of phthalate esters in diapers and wipes using gas chromatography. Microchem J. 2018;142:359–66. Available from: https://doi.org/10.1016/j.microc.2018.07.015
- Ishii S, Katagiri R, Minobe Y, Kuribara I, Wada T, Wada M, et al. Investigation of the amount of transdermal exposure of newborn babies to phthalates in paper diapers and certification of the safety of paper diapers. Regul Toxicol Pharmacol. 2015;73(1):85–92. Available from: https://doi.org/10.1016/j.yrtph.2015.06.010
- Park CJ, Barakat R, Ulanov A, Li Z, Lin PC, Chiu K, et al. Sanitary pads and diapers contain higher phthalate contents than those in common commercial plastic products. Reprod Toxicol. 2019;84:114–21. Available from: https://doi.org/10.1016/j.reprotox.2019.01.005
- Zhang SX, Chai XS, Jiang R. Accurate determination of residual acrylic acid in superabsorbent polymer of hygiene products by headspace gas chromatography. J Chromatogr A. 2017;1485:20–3. Available from: https://doi.org/10.1016/j.chroma.2017.01.023
- Hamasaki T. Simultaneous determination of organotin compounds in textiles by gas chromatography-flame photometry following liquid/liquid partitioning with tert-butyl ethyl ether after reflux-extraction. Talanta. 2013;115:374–80. Available from: https://doi.org/10.1016/j.talanta.2013.04.041
- Šmajgl D, Obhodaš J. Occurrence of tin in disposable baby diapers. X-Ray Spectrom. 2015;44(4):230–2. Available from: https://doi.org/10.1002/xrs.2609
- Na YR, Kwon HJ, Cho HN, Kim HJ, Park YK, Park SA, et al. Formaldehyde monitoring of hygiene products in domestic market. J Food Hyg Saf. 2020;35(3):225–33. Available from: https://doi.org/10.13103/jfhs.2020.35.3.225
- Liu W, Xue J, Kannan K. Occurrence of and exposure to benzothiazoles and benzotriazoles from textiles and infant clothing. Sci Total Environ. 2017;592:91–6. Available from: https://doi.org/10.1016/j.scitotenv.2017.03.090
- Makoś-Chełstowska P, Kurowska-Susdorf A, Płotka-Wasylka J. Environmental problems and health risks with disposable baby diapers: Monitoring of toxic compounds by application of analytical techniques and need of education. TrAC Trends Anal Chem. 2021 Oct 1. Available from: https://doi.org/10.1016/j.trac.2021.116408
- Kwon YS, Choi SG, Lee SM, Kim JH, Kim SG, Lee DY, et al. Improved method for the determination of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) in sanitary napkins. Anal Lett. 2020;53(2):273–89. Available from: https://doi.org/10.1080/00032719.2019.1647226
- Rodriguez KJ, Cunningham C, Foxenberg R, Hoffman D, Vongsa R. The science behind wet wipes for infant skin: Ingredient review, safety, and efficacy. Pediatr Dermatol. 2020;37(3):447–54. Available from: https://doi.org/10.1111/pde.14112
- Kuller JMM, Zukowsky K. Infant skin care products: What are the issues? Adv Neonatal Care. 2016;16(5 Suppl):S3–12. Available from: https://journals.lww.com/advancesinneonatalcare/abstract/2016/10001/infant_skin_care_products__what_are_the_issues_.2.aspx
- Cahill LJ, Toholka WR, Nixon RL. Contact allergy in children. Med J Aust. 2014;200(4):213.
- Celeiro M, Lamas JP, Garcia-Jares C, Llompart M. Pressurized liquid extraction–gas chromatography–mass spectrometry analysis of fragrance allergens, musks, phthalates and preservatives in baby wipes. J Chromatogr A. 2015 Mar 6;1384:9–21. Available from: https://doi.org/10.1016/j.chroma.2015.01.049
- Rocha BA, Bocato MZ, Latorraca EF, Ximeneza JPB, Barbosa F. A survey of parabens in commercial baby wipes from Brazil and estimation of daily exposure. Quim Nova. 2020 Apr 1;43(4):442–6. Available from: https://doi.org/10.21577/0100-4042.20170494
- Brand W, Boon PE, Hessel EVS, Meesters JAJ, Weda M, Schuur AG. Exposure to and toxicity of methyl-, ethyl- and propylparaben: A literature review with a focus on endocrine-disrupting properties [Internet]. 2018. Available from: https://www.rivm.nl/en
- Ishiwatari S, Suzuki T, Hitomi T, Yoshino T, Matsukuma S, Tsuji T. Effects of methyl paraben on skin keratinocytes. J Appl Toxicol. 2007;27:1–9. Available from: https://doi.org/10.1002/jat.1176
- Komamura H, Dor T, Inui S, Yoshikawa K. [No title available]. 1997.
- Singh H, Poulos A. A comparative study of stearic and lignoceric acid oxidation by human skin fibroblasts. Arch Biochem Biophys. 1986;250(1):171–9. Available from: https://doi.org/10.1016/0003-9861(86)90714-9
- Sushmitha C. Baby care–baby care products and harmful ingredients used in baby products. Asian J Pharm Tech Res. 2019. Available from: https://ajptr.com/assets/upload/publish_article/AJPTR_96004.pdf
- Magon S. ISSN 2347-954X (Print) Use of Talcum Powder on Infants and Toddler [Internet]. Vol. 2, Scholars Journal of Applied Medical Sciences (SJAMS. 2014. Available from: https://saspublishers.com/article/7025/download/
- Bergfeld WF, Donald FACP, Belsito V, Hill RA, Klaassen CD, Liebler DC, et al. Safety assessment of talc as used in cosmetics. Final Report. 2013 Apr 12.
- Rohl AN, Langer AM, Selikoff IJ, Tordini A, Klimentidis R, Bowes DR, et al. Consumer talcums and powders: Mineral and chemical characterization. J Toxicol Environ Health. 1976;2(2):255–84. Available from: https://doi.org/10.1080/15287397609529432
- Tran TH, Steffen JE, Clancy KM, Bird T, Egilman DS. Talc, asbestos, and epidemiology: Corporate influence and scientific incognizance. Epidemiology. 2019;30:783–8. Available from: https://journals.lww.com/epidem/fulltext/2019/11000/talc,_asbestos,_and_epidemiology__corporate.2.aspx
- Gordon RE, Fitzgerald S, Millette J. Asbestos in commercial cosmetic talcum powder as a cause of mesothelioma in women. Int J Occup Environ Health. 2014;20(4):318–32. Available from: https://doi.org/10.1179/2049396714Y.0000000081
- Hollinger MA. Pulmonary toxicity of inhaled and intravenous talc. Toxicol Lett. 1990;52:121–7. Available from: https://doi.org/10.1016/0378-4274(90)90145-C
- Karwowski MP, Morman SA, Plumlee GS, Law T, Kellogg M, Woolf AD. Toxicants in folk remedies: implications of elevated blood lead in an American-born infant due to imported diaper powder. Environ Geochem Health. 2017;39(5):1133–43. Available from: https://link.springer.com/article/10.1007/s10653-016-9881-6
- Randive DS, Bhinge SD, Jadhav NR, Bhutkar MA, Shirsat MK. Carbon based kajal formulations: Antimicrobial activity and feasibility as a semisolid base for ophthalmics. J Pharm Res Int. 2020;32:62–74. Available from: https://journaljpri.com/index.php/JPRI/article/view/1461
- Chhablani J. Combined photodynamic therapy and intravitreal ranibizumab as treatment for extrafoveal choroidal neovascularization associated with age-related macular degeneration. Oman J Ophthalmol. 2010;3(2):99–100. Available from: https://doi.org/10.4103/0974-620x.64241
- Tiffany-Castiglioni E, Barhoumi R, Mouneimne Y. Kohl and surma eye cosmetics as significant sources of lead (Pb) exposure. J Local Glob Health Sci. 2012;2012(1). Available from: http://dx.doi.org/10.5339/jlghs.2012.1
- Mustehasan, A. M.; Naushin, S.; Urooj, M.; Husain, G. M. Overview of Sang-e-Surma (Antimony Sulphide or Lead Sul-Phide): A Mineral Origin Unani Drug; 2022; Vol. 7.
- Al-Ashban RM, Aslam M, Shah AH. Kohl (surma): A toxic traditional eye cosmetic study in Saudi Arabia. Public Health. 2004;118(4):292–8. Available from: https://doi.org/10.1016/j.puhe.2003.05.001
- Al-Saleh I, Mustafa A, Dufour L, Taylor A, Hiton R. Lead exposure in the city of Arar, Saudi Arabia. Arch Environ Health. 1996;51(1):73–82. Available from: https://doi.org/10.1080/00039896.1996.9935997
- Sathyanarayana S, Karr CJ, Lozano P, Brown E, Calafat AM, Liu F, et al. Baby care products: Possible sources of infant phthalate exposure. Pediatrics. 2008;121(2):e260–8. Available from: https://doi.org/10.1542/peds.2006-3766
- Robinson VC, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Marks JG, et al. Final report of the amended safety assessment of sodium laureth sulfate and related salts of sulfated ethoxylated alcohols. Int J Toxicol. 2010;29(4):151S–161S. Available from: https://doi.org/10.1177/1091581810373151
- Nikle A, Ericson M, Warshaw E. Formaldehyde release from personal care products: Chromotropic acid method analysis. Dermatitis. 2019;30(1):67–73. Available from: https://doi.org/10.1097/der.0000000000000434
- Backe WJ. A novel mass spectrometric method for formaldehyde in children’s personal-care products and water via derivatization with acetylacetone. Rapid Commun Mass Spectrom. 2017;31(12):1047–56. Available from: https://doi.org/10.1002/rcm.7874
- de Groot AC, Flyvholm MA, Lensen G, Menné T, Coenraads PJ. Formaldehyde-releasers: relationship to formaldehyde contact allergy. Contact Dermatitis. 2009;61:63–85. Available from: https://doi.org/10.1111/j.1600-0536.2009.01582.x
- Symanzik C, Weinert P, Babić Ž, Hallmann S, Havmose MS, Johansen JD, et al. Skin toxicity of selected hair cosmetic ingredients: A review focusing on hairdressers. Int J Environ Res Public Health. 2022 Jul 1;19(13):7588. Available from: https://doi.org/10.3390/ijerph19137588
- Lim TY, Poole RL, Pageler NM. Propylene glycol toxicity in children. J Pediatr Pharmacol Ther. 2014 Oct-Dec;19(4):277–82. Available from: https://doi.org/10.5863/1551-6776-19.4.277
- Wang J, Liu Y, Kam WR, Li Y, Sullivan DA. Toxicity of the cosmetic preservatives parabens, phenoxyethanol and chlorphenesin on human meibomian gland epithelial cells. Exp Eye Res. 2020;196:108057. Available from: https://doi.org/10.1016/j.exer.2020.108057
- Cullen MR, Balmes JR, Robins JM, Smith GJW. Lipoid pneumonia caused by oil mist exposure from a steel rolling tandem mill. Am J Ind Med. 1981;2:51–8. Available from: https://doi.org/10.1002/ajim.4700020109
- Spickard A, Hirschmann JV. Exogenous lipoid pneumonia. Chest. 1994;154:686–92. Available from: https://pubmed.ncbi.nlm.nih.gov/8129503/
- Pirow R, Blume A, Hellwig N, Herzler M, Huhse B, Hutzler C, et al. Mineral oil in food, cosmetic products, and in products regulated by other legislations. Crit Rev Toxicol. 2019;49:742–89. Available from: https://doi.org/10.1080/10408444.2019.1694862