More Information
Submitted: November 07, 2024 | Approved: November 11, 2024 | Published: November 13, 2024
How to cite this article: Kearse K, Ligaj F. Scientific Analysis of Eucharistic Miracles: Importance of a Standardization in Evaluation. J Forensic Sci Res. 2024; 8(1): 078-088. Available from: https://dx.doi.org/10.29328/journal.jfsr.1001068
DOI: 10.29328/journal.jfsr.1001068
Copyright License: © 2024 Kearse K, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Eucharistic miracles; Communion wafers; Forensics; Non-human DNA
Scientific Analysis of Eucharistic Miracles: Importance of a Standardization in Evaluation
Kelly Kearse* and Frank Ligaj
and Frank Ligaj
Knoxville Catholic High School, 9245 Fox Lonas Road, Knoxville, TN 37923, USA
*Address for Correspondence: Dr. Kelly Kearse, Knoxville Catholic High School, 9245 Fox Lonas Road, Knoxville, TN 37923, USA, Email: [email protected]
Numerous instances of consecrated communion wafers turning into human tissue and blood have been reported throughout history and the contemporary international media, referred to as Eucharistic miracles. Various suggestions have been put forth to explain such phenomena, ranging from miraculous to natural. Here, a novel demonstration is provided showing that the appearance of a bleeding host can occur by placing ordinary, non-consecrated wafers under similar conditions as described for many of these events. Using basic forensic methods, distinctions between ensuant reddish areas and genuine blood were noted. In previous studies with miracle wafers, isolated DNA was resistant to amplification with human-specific primers, which has been attributed to its divine nature. The current study shows that multiple types of non-human DNA existed in unconsecrated wafers, providing an alternative explanation for such findings. Finally, a minimal protocol of scientific examination is outlined to aid in the standardization of such investigations in the future, including a distinctive approach to authenticate the genuine shared origin of such occurrences.
Four of the most scientifically studied Eucharistic miracles have occurred in the twenty-first century, and numerous, lesser-studied events may be found throughout the contemporary media [1-9]. For many of these instances a host (consecrated communion wafer) was typically found unattended in the church and in compliance with Church protocol, placed in a vessel containing water to dissolve prior to special disposal. The wafers were often described as dusty or dirty. A week or so after being placed in water, bright red spots became apparent, with the appearance of “fresh blood”, prompting further investigation.
Although not regularly verified, contamination with the bacteria Serratia marcescens has been suggested as a causative agent for the appearance of fresh blood on communion bread, discovered by Bartholomeo Bizio, an Italian pharmacist, in 1817 [10]. The bright red color results from the pigment prodigiosin and/or other related materials. As detailed by Bennett and Bentley, when such bacterial colonies mature “they dissolve into a fluid and viscous state with a mucilaginous appearance and an uncanny resemblance to blood”, [11]. Numerous other bacteria and fungi may also produce reddish products [12,13]. Below, a brief narrative of six of the most well-publicized Eucharistic miracles is provided, with a particular focus on the methodology used in their scientific analysis.
Lanciano, Italy 7th century
The miracle of Lanciano was evaluated by Linoli in 1971, who published his results in the Italian medical journal Quaderni Sclavo di Diagnostica [14]. Although somewhat dated, a brief review of this report is important as many modern events often refer to this study as a type of corroborating evidence.
According to tradition, as the priest prayed the prayer of consecration, the communion bread changed into living flesh and the wine changed into living blood. Upon examination in the 1970s, the sample was found to contain whitish specs (fungus) and showed “conspicuous accumulations of spores and of hypha of hyphomyces”, [4,14]. The blood had a consistency that was “uniformly hard, so that only with strong pressure from the cutting edge was it possible to detach some small parts with difficulty” [4,14]. Primary Teichman and Takayama tests, which evaluate the presence of hemoglobin, were both negative. Because initial attempts to test for the presence of human blood using the Ochterlony method (an immunodiffusion technique) were problematic, a less sensitive technique, zonal diffusion, was used [4,14]. The fidelity of such results is somewhat unknown, however, given the difficult nature of obtaining a sample with a large degree of hardness and potentially limited solubility, analyzed using a diffusion-based technique. Potential issues with cross-reactivity are also a concern (see below). In a recent book on the scientific nature of Eucharistic miracles, “A Cardiologist examines Jesus”, Serafini states that “it is qualitatively unquestionable that the miracle of Lanciano contains true human blood.” [4]. Unfortunately, however, it cannot be declared that the results are specific for human blood as very limited demonstration is given regarding the potential cross-reactivity of the antisera used with only two other species tested, bovine and rabbit [14]. Indeed, this study relies on a polyclonal antibody preparation made via a relatively unrefined immunization using whole human blood; such preparations would be expected to be potentially cross-reactive with blood from other species. The preliminary histological evaluation suggested that the tissue was cardiac in origin [14] and follow-up studies led to the conclusion that both endocardium and adipose tissue were present, as well as arterial and venous structures [4]. ABO blood typing was performed with a result of type AB; however, doubts have been raised regarding the validity of such results as bacteria also express AB antigens [4,15]. As recently reviewed, more contemporary techniques that distinguish between bacterial and human AB antigens may help clarify the accuracy of the findings of shared AB expression among various Eucharistic miracles and Christian relics [15].
The Lanciano investigation is commendable in that it represents the first major scientific attempt to objectively evaluate the physical properties of a Eucharistic miracle in any detail. It is unfortunate that in subsequent years fraudulent activity (by others) would become associated with these findings. It was reported that subsequent tests (over 500 of them!) were performed on these samples, verifying the physical presence of true flesh and true blood [4,16,17]. The experiments were purportedly conducted by a medical commission from the World Health Organization (WHO) and the United Nations (UN); the results were said to have been publicized in New York and Geneva and contained within a large summary report. Several years ago, we (and others) searched in vain for any results or documentation from this report and contacted both the WHO and UN, who had no records of any investigation during that period. More recently, the Italian physician Dr. Franco Serafini uncovered the location of this report, residing within a safe inside a monastery in Lanciano. He requested permission to examine the documents and much to his dismay, found that someone had attached a front and back page about Lanciano to hundreds of unrelated pages of tests on Egyptian mummies. After just a few minutes of examination, it was apparent that the report was a “terrible fraud, a terrible hoax, very sad”, [4,17].
Buenos Aires, Argentina 1996
In this case, a host was found discarded near a candlestick holder toward the back of the church; the priest’s first inclination was to consume it but because it was so dirty, opted to place the wafer in water for future disposal according to Church protocol [2-4,17]. Approximately eleven days later a reddish color was observed on the host; the sample was kept in water for several more years. In 1999, scientific investigation began under the leadership of Ricardo Castanon Gomez, a clinical psychologist, who specialized in brain chemistry. Histological samples were sent out to several experts for evaluation, particularly cardiologists and pathologists who scored the sample as either muscle or heart cells containing infiltrating white blood cells. Dr. Fred Zugibe, a pathologist at Columbia University specializing in cardiology reported that the sample was from “tissues of the heart, undergoing degenerative changes of the myocardium and these changes are due to the fact that the cells are inflamed, and it is the left ventricle”, [2-4]. These are remarkable results and given their potential importance, several things should be noted: First, the primary experts that were sought out were pathologists or cardiologists, indicating that the assumption had likely been made that this was human (cardiac) tissue. A more objective approach would have solicited additional input from scientists of other disciplines, including microbiologists and mycologists. Second, it would have been more valuable to conduct the study in a true blind fashion and mix in with the experimental slides various control slides prepared in the same manner. Although it is reported that Zugibe did not know the nature of the sample he examined, the presence of the camera crew might have alerted him that these did not likely originate from an ordinary John Doe.
DNA studies were performed, and the test results were relatively well documented and available [2-4]. In describing these results, many speakers and authors report that such tests verified the presence of human DNA, which was unable to be amplified, hinting at a divine nature of the sample [2-4,17,18]. As noted by Trasancos, this is an over-exaggeration of the findings [17]. The target DNA to be amplified included multiple Short Tandem Repeat regions (STRs) which are typically used in comparative analysis for identity determination. The report states that “a very low concentration of human DNA was recovered” and that the sample “contained high molecular weight (good quality) DNA of non-human origin.” The very low concentration of human DNA that was recovered is characteristic of contaminating DNA, the likely result of someone touching the sample. This is a very different situation than having an abundant amount of human DNA, which could not be amplified. In reading the narrative of the incident, it is clear that the sample was handled by multiple persons, understandably without Personal Protective Equipment (PPE). The report states that extraction controls and positive and negative PCR controls were run with each set of PCR reactions, but no specific mention is made of a spike control; a spike control is useful in situations where it is suspected that an inhibitory substance might be present, helping to further explain the results. It is also important to consider other possible non-miraculous sources of DNA that could exist in the sample, such as bacteria, fungi, and plants (wheat). Indeed, wheat DNA has been shown to survive the baking process under relatively high temperatures and exposure conditions and to be successfully amplified by PCR [19]. Given the suggested involvement of microorganisms in descriptions of such miracles in the past, it would have been prudent to also consider the DNA contribution from such microorganisms.
Tixtla, Mexico 2006
Here, a nun was distributing communion when she noticed a reddish substance similar to blood exuding from a host. The priest was notified, and the wafer was reserved in a separate place. Three years later, a scientific investigation began under the direction of Ricardo Gomez, who had performed similar duties in the Buenos Aires incident [5]. Typing experiments revealed the sample tested positive for AB and immunochromatography experiments were reported as positive for human Hemoglobin (Hb). In the type of assay that was performed for hemoglobin, the sample is loaded into a chamber where it will migrate into a region containing antibodies (anti-Hb), which are then carried downstream to encounter immobilized antibodies on a test line (to see if the sample is positive), and a control line (to demonstrate that the assay was functional); many will be familiar with similar home type tests to evaluate pregnancy or COVID exposure. A positive finding for hemoglobin suggests that something extraordinary may have happened and deserves further scrutiny, especially related to the details of the technique. Cross-reactivity of antibodies with hemoglobin from species other than humans is typically not a concern with such diagnostic tests on known persons; however, in this scenario, it is important to verify that the antibodies used are indeed specific for human blood. It is also critical to emphasize that such tests are optimized for the use of liquid blood which has a specific consistency/density and pH. Because certain Eucharistic events have been attributed to bacteria that produce a viscous, mucilaginous fluid [10,11], it is possible that the increased viscosity of such a sample could result in nonspecific antibody binding, resulting in a false positive. As little to no details were provided for such tests, other than the result, it is unclear if differences in fluidity might be a factor. Immunochromatography techniques have also been shown to be significantly affected by the pH of the sample [20,21]; it is unknown if this was a consideration in the interpretation of the results. In such immunochromatography tests it would have been suitable to run control (and contaminated) wheat wafer samples in parallel. As alluded to above, extraordinary events require that scientists include consideration of all available ordinary means in their investigation.
Histological tests were performed (again spearheaded by Gomez), consulting multiple pathologists and cardiologists. Similar interpretations were reported as before, in cardiac tissue with the presence of red blood cells and white blood cells, including macrophages, neutrophils, and basophils [4,5]. One pervading issue in many of these histological reports is that they are based on visual inspection alone. While this may be suitable in the normal routine of examining specimens from persons about which considerable information is known, identification of certain cell types or tissues in cases such as these requires accompanying studies with cell-specific markers. To this end, the investigators reported that the samples were positive for glycophorin A (a red blood cell marker) but tested negative using probes for muscle-desmin and myosin (both muscle-specific). The latter result was unexpected given the proposed nature of the material. The glycophorin A result is interesting, although as before, no specificity controls were presented. As discussed above, it is paramount to include both negative and positive controls that ensure that the results are unambiguously scientifically valid. Staining with anti-glycophorin A may be relatively routine in the examination of cardiac tissue from known human samples, but one wonders if any nonspecific binding would occur in control wafer samples. The presence of plant cells in the Tixtla sample was acknowledged, “recognized every now and then in the background” [4,5].
Similar to the Buenos Aires event, DNA studies were done with the conclusion that the DNA was “completely degraded and fragmented”. Serafini imparts that this once again is in keeping with the idea that “the genetic material escapes genetic probes and does not lend itself to be recognized”, [4]. The possibility of non-human DNA being present was not considered. ABO typing was performed by two separate groups, using both agglutination and immunofluorescent techniques, and a result of AB was given, although no demonstration of specificity controls in the sample was provided. As previously emphasized, the contribution of AB antigens by bacteria cannot be excluded with either serological technique and should be taken into consideration in the interpretation of the results. Lastly, the sample was reported to be Rh negative; ironically it has been overlooked that this is in opposition to the concept of a universal recipient phenotype, proposed on theological principles [4].
Sokolka, Poland 2008
A host was noticed on the steps of the altar by a communicant, and because the wafer appeared dirty, the priest immersed it in a container of water. Later the same day, the contents were transferred to a larger container, and more water was added. A week later, it was noticed that the host had not dissolved and was covered by a red stain, approximately 1 x 1.5 cm [3,4]. The water was untainted by the color, an odd finding if dried blood or bleeding were involved (see below). Seventeen days after the initial observation, the priest separated the remaining bread and red clot from the water and laid it on a corporal (white cloth). The cloth was then transferred to a monstrance (a container for the display of consecrated hosts) and placed inside the tabernacle. Approximately three months later, samples were taken for histological analysis, performed by two different investigators. In this study, both light microscopy and transmission electron microscopy were employed; the conclusion was that the sample was myocardial tissue, particularly from someone showing signs of physiological stress.
Legnica, Poland 2013
A consecrated host that had just been dipped in wine accidentally fell to the ground, and according to procedure, was placed in a metal chalice with tap water to dissolve. Approximately eleven days later it was noticed that a small crescent-shaped portion had detached itself and was turning red [3,4]. Three weeks after this observation, very small samples were removed for scientific analysis. Conclusions from this investigation include the presence of cardiac muscle “displaying changes that often accompany a state of agony”. These results were based on visual observation only as “the considerable tissue degradation prevented definitive immunohistochemical confirmation” of the tissue type [4].
Sufficient fragments of both nuclear and mitochondrial (mt) DNA were present, allowing the proof of “the human origin of the tissue beyond doubt”, [4]. While no details were provided, it is unlikely that DNA samples, particularly mitochondrial (mt) DNA, were compared with profiles of all individuals known to be in contact with the material. This is an important consideration in any investigation of this nature, which should be addressed. Microscopic examination showed that bacteria were present but “only represented a negligible local contamination”, and it was reported that no bacteria existed that were known to produce coloring substances, like Serratia marcescens. Additionally, it was stated that there was “no significant fungal contamination”.
Tyrol, Austria 2016
The details of this event were recently reported in the Annals of Case Reports by Galvin Publishers [22]. This is only the second miracle-type event that has been incorporated into the scientific literature, the first being the Lanciano occurrence, published in the early 1970s (see above). Similar to previous incidents, a host was found on the floor of the church, which was placed inside a container containing water; fourteen days later it was noticed that red spots were present. After four years, samples were removed for analysis, involving primarily microscopic examination. Staining showed that gram-positive rods were present together with a mixed bacterial flora. Myriads of hyphae and spores were observed, some of them pigmented. Eosinophilic roundish structures were noted, which in this case were interpreted as fungal spores [22].
Taken together, these reports provide descriptions of shared features that have been ascribed to a common origin, most notably the suggestion that genuine blood seeped from consecrated wafers. In the scientific analysis of Eucharistic miracles, it is interesting to speculate what might happen if a non-consecrated communion wafer were treated in a similar manner as described for many of the above occurrences. Surprisingly, we were unable to find a single instance where such a basic experiment had been performed. Here, we provide a novel demonstration that the appearance of a bleeding host can occur by placing non-consecrated wafers under similar conditions as described for many of these events. Using several fundamental scientific techniques, major differences between fresh blood and ensuant reddish areas were denoted. Multiple sources of non-human DNA were shown to be present in unconsecrated wafers, providing an explanation for previous failed amplification attempts with miracle wafers. Finally, a minimum protocol is suggested to aid in the standardization of evaluation and scientific validation of such cases in the future.
Non-consecrated wheat wafers and culture conditions
Wheat communion wafers were purchased from the Cavanaugh Altar Breads company (Greenville, RI), a common supplier for many parishes in the United States. Wafers were left in a dusty and dark corner for several days; samples were then placed in approximately 200 ml of tap water in plastic 16-ounce Solo cups (Lakeforest, IL) and cultured for 7-10 days at ambient temperature and humidity.
Blood studies and staining
Fresh blood was obtained from healthy volunteers using the finger stick method. Fifty to one hundred microliters were typically used in each analysis. For blood demonstration two standard, established techniques were employed: Wright-Giemsa staining, routinely used in differential blood counts [23] and spectroscopy methods, used in the evaluation of hemoglobin, a signature diagnostic molecule of blood [24,25]. Wright-Giemsa staining was done using a Volu Sol Dip-Stain kit (Salt Lake City, UT); lactophenol cotton blue fungal stain was obtained from Dawn Scientific Inc. (Metuchen, NJ). Ultraviolet light fluorescence was measured using a 365 SK68 UV flashlight from Darkness Beam Company (London, UK). Spectroscopy was done using a Pasco 2600 portable wireless spectrometer (Hudson, OH).
Serratia marcescens
Purified Serratia marcescens cultures were purchased from Carolina Biological Supply Company (Burlington, NC) and grown on Evviva Sciences nutrient agar plates (St. Fremont, CA). Plate cultures were swabbed with sterile inoculation loops and transferred to wheat wafers placed on damp paper towels and incubated at ambient temperature and humidity. Reddish growth on wafers was apparent within a 24-48 hour time period.
DNA analysis
DNA samples were extracted using Qiagen’s QIAamp PowerFecal Pro DNA Kit (Venlo, the Netherlands) according to the manufacturer’s instructions.
Wheat primers Dgas44F: (5’CTTCTACGGGTCAGGGCAC3’) and Dgas44R: (5’CTAATGCCCCTGCGGCTTAA3’) were diluted to 2uM. 16S primers 341F: (5’CCTACGGGNGGCWGCAG3’) and 785R: (5’GACTACHVGGGTATCTAATCC3’) were diluted to 1.5uM. ITS primers ITS3NGS3 (5’CACCGATGAAGAACGCAG3’) and ITS4NGR (5’TCCTSCGCTTATTGATATGC3’) were diluted to 1.5uM.
PCR with the wheat primers was performed with the dry wafer, wet wafer in sterile water, and wet wafer in lysis buffer samples with 12.5uL of Kapa HiFi Master Mix, 5uL of each primer, and 5uL of DNA, which is double the standard amount to improve amplification.
PCR with the 16S and ITS primers was performed on the wet colony, and both dry colony samples using 12.5 uL of Kapa HiFi Master Mix (Roche, Indianapolis, IN), 5uL of each primer, and 2.5 uL of DNA. All PCR products were visualized on a 2% agarose gel. In preparation for Sanger sequencing through Eurofins, PCR products were cleaned with 2uL of ExoSap (Thermo Fisher, Asheville, NC) for each 5 uL of PCR product. This reaction was cycled at 37 ℃ for 15 minutes and 80 ℃ for 15 minutes. Sanger sequencing was done via Eurofins Genomics. Sequencing products were aligned using MEGA (Molecular Evolutionary Genetics Analysis) Software and sequences were compared to sequences in Genbank using BLAST.
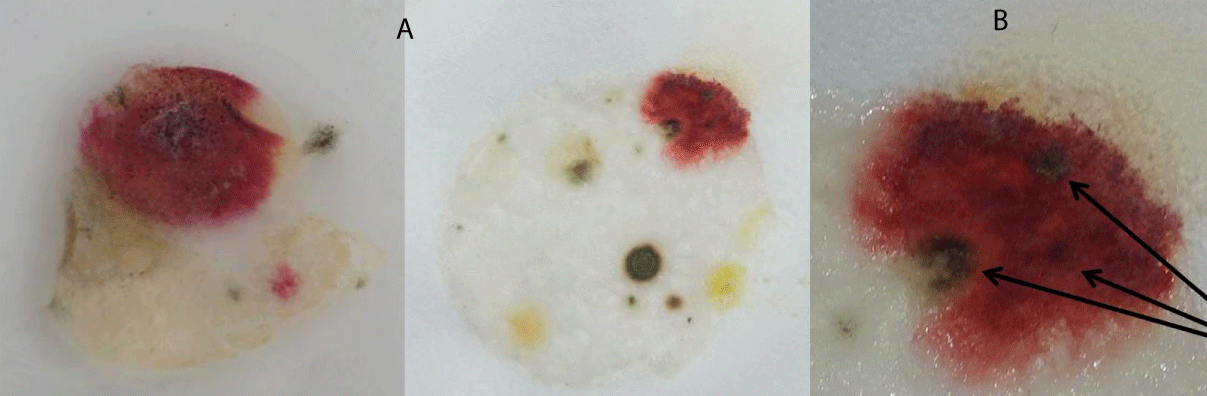
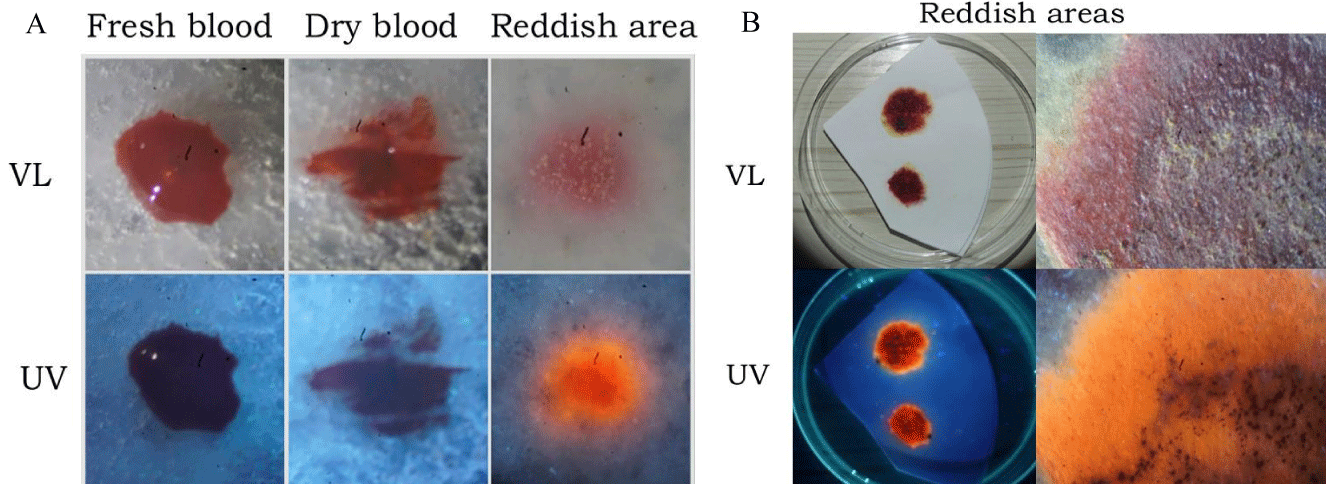
In our initial studies, we placed non-consecrated wheat communion wafers in various areas on or near the floor for several days and then transferred them to containers containing approximately 200 milliliters of tap water to reproduce what had been described in various miracle reports. Samples were kept indoors, undisturbed, at ambient temperature and humidity, and away from direct sunlight. In approximately 15% of the cases, a bright red area was noticed growing on the remaining wafer portion some 7-10 days later. Representative results are presented in Figure 1 (Figure 1A). When wafers were removed from the water and placed on a piece of filter paper to dry, the bread was observed to tightly cling to the substrate after drying. If one compares such images with those of various Eucharistic miracles, for example, Sokolka, 2008 [26], similar features are apparent, including certain darkened areas (Figure 1B, arrow).
Figure 1: Reddish spots resembling blood were observed on non-consecrated wafers cultured in water. (A) Representative results of non-consecrated wafers cultured in water for approximately 7-10 days. (B) Arrows indicate a darkened area identified as sporodochia, characteristic of fungus.
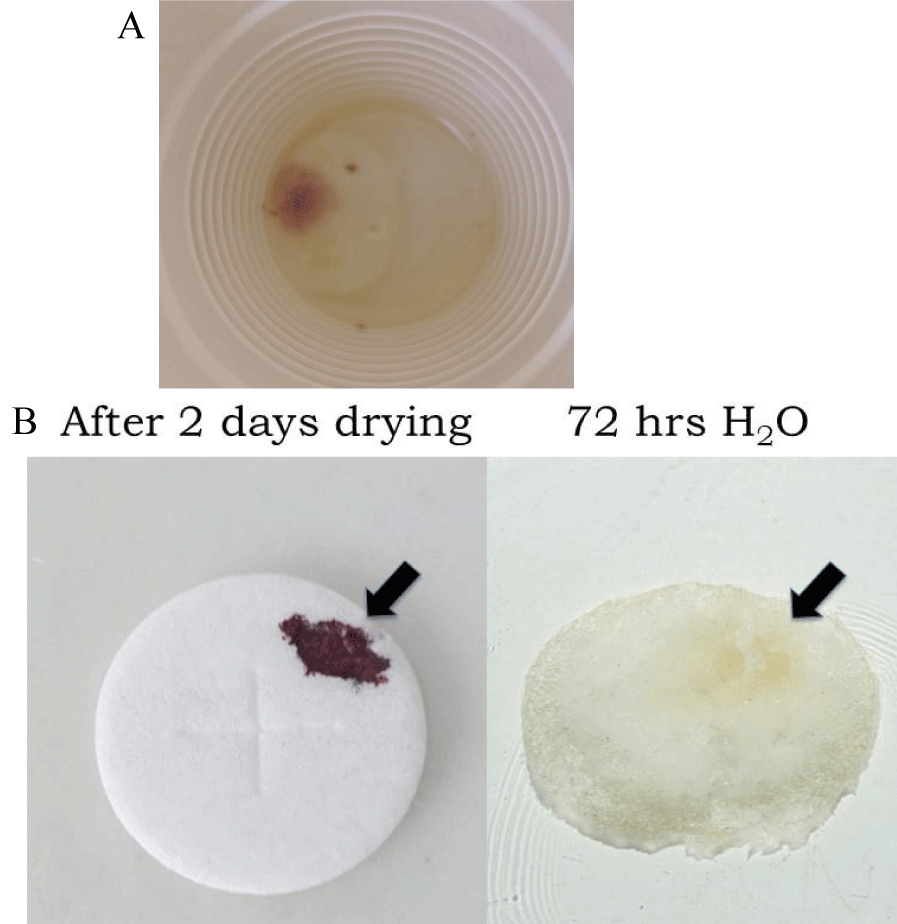
In the study of Eucharistic miracles, it has been reported that the water remained untainted one week later after the host was immersed and the red stain became apparent [3,4]. This is intriguing as fresh blood, or freshly dried blood is readily dissolved in water and many aqueous-based solvents [27]. In the current study using non-consecrated wafers containing reddish areas, it was noted that the water remained untainted as well (Figure 2A). As shown in Figure 2B, when a small amount of blood was placed on a wafer, allowed to dry for two days, and then placed in water, within 72 hours the bloodstain was fully solubilized (Figure 2B). It was also noted that unlike freshly dried blood, which was readily solubilized in aqueous solvents, such as 1% NP-40 or 95% methanol, the reddish areas on experimental wafers remained relatively insoluble (data not shown). These results demonstrate that reddish areas on water-cultured wafers showed different solubility properties relative to freshly dried blood.
Figure 2: Solubilization properties of reddish growths and genuine blood. (A). Non-consecrated wafers were placed in water for 7 days. Despite the appearance of reddish growths on portions of the wafer, the water remained untainted. (B). Blood was added to non-consecrated wafers and allowed to dry overnight; samples were then placed in water and examined 72 hours later.
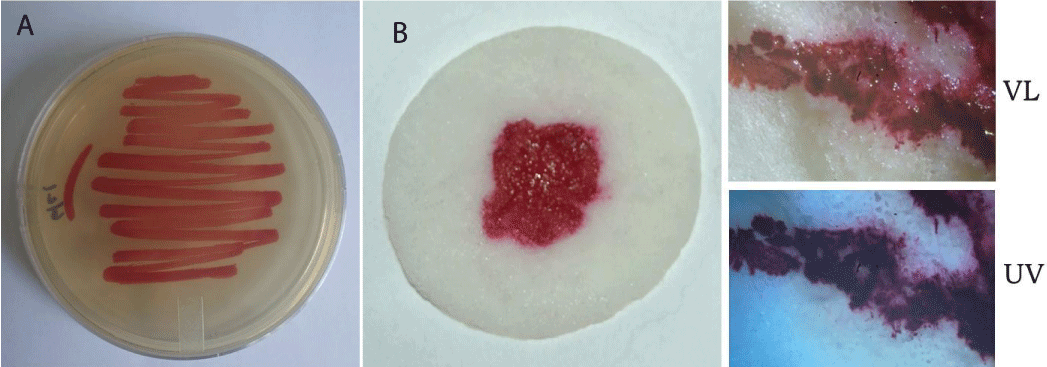
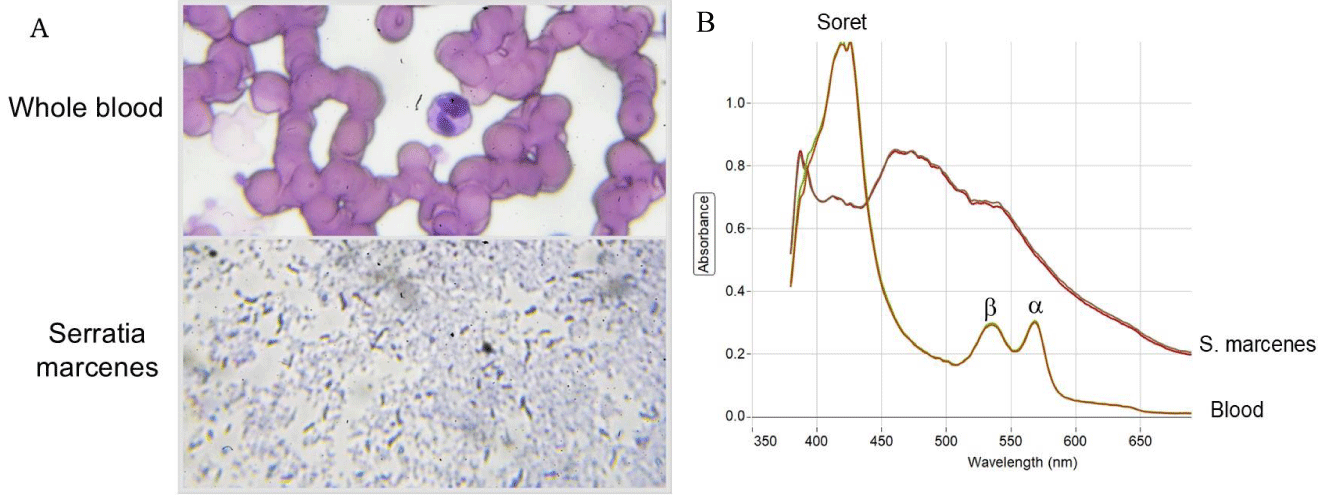
As Serratia marcescens has been frequently suggested as a potential causative agent in Eucharistic miracle events, wafers were inoculated with a purified culture of this bacteria (Figures 3A,B). Within 24-48 hours a noticeable reddish region corresponding to the treated area was visible (Figure 3B), having a similar appearance to that of fresh blood; the likenesses with certain images of reported Eucharistic miracles are evident [3,4]. Like whole blood, reddish areas containing Serratia marcescens did not fluoresce under ultraviolet light (Figure 3B); however, blood was easily distinguished from Serratia marcescens growth by Wright’s staining (Figure 4A) and spectroscopy (Figure 4B), two well established, basic techniques.
Figure 3: Non-consecrated wafers inoculated with Serratia marcescens show the appearance of blood. (A) Purified culture of Serratia marcescens grown on agar plates. (B) Non-consecrated wafers were inoculated with Serratia marcescens and cultured for 24-48 hours on a wet surface. The resemblance to blood is apparent. Samples were examined under visible light (VL) or ultraviolet light (UV). Similar to whole blood, S. marcescens growth does not exhibit fluorescence under UV.
Figure 4: Standard staining and spectroscopy methods distinguish blood from Serratia marcescens growth. (A) Microscopic examination of Wright’s staining of whole blood (top) and Serratia marcescens growth (bottom). (B) Spectroscopy analysis of whole blood and Serratia marcescens growth. The presence of distinct, standard peaks (Soret band and , peaks of hemoglobin), characteristic of blood are marked.
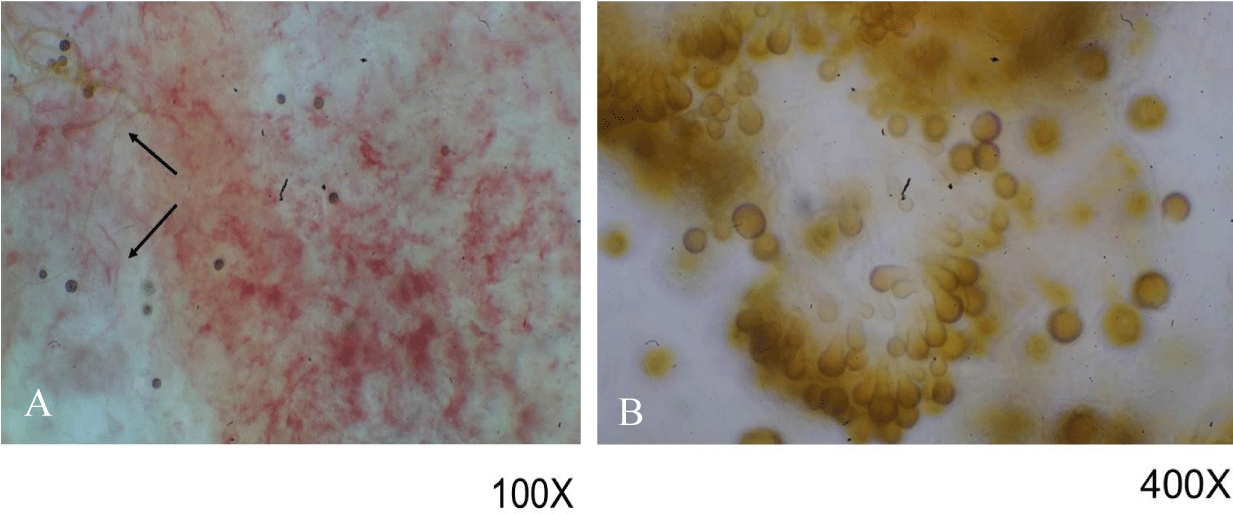
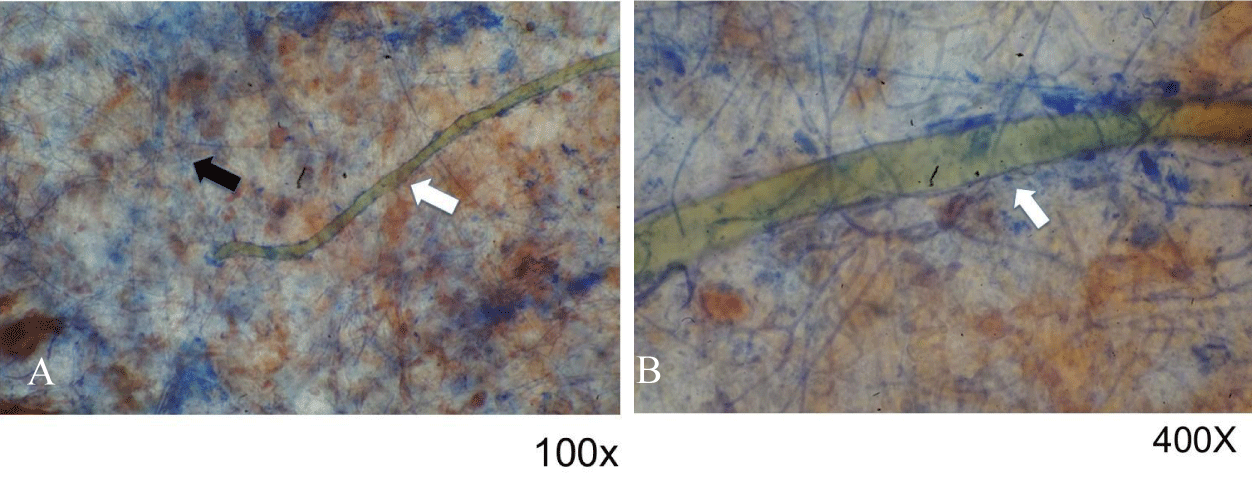
Unlike what was observed with wafers inoculated with Serratia marcescens, the examination of non-consecrated wafers (described above) under ultraviolet light showed a distinct reddish-orange fluorescence (Figures 5A,B). Consultation with various microbiologists indicated that the reddish growths were likely fungal in origin. As noted previously, reddish areas in non-consecrated wafers cultured in water showed certain darkened areas (Figure 1B), which in our studies were identified by fungal experts as sporodochia; such regions appear dark due to the presence of mature conidia, chlamydospores, a resting state that aids in spore survival under harsh conditions. These findings were verified by microscopic examination, which also showed that fungal hyphae were present (Figure 6A); higher magnification showed the existence of immature conidia and chlamydospores, which are lighter in color (Figure 6B). Fungal infestation was verified by staining with the fungal stain lactophenol cotton blue which is specific for chitin (Figures 7A,B). Chitin is not present in wheat wafers and does not stain blue under these conditions, a wafer strand is indicated by the arrow in Figures 7A,B. Taken together, these data indicate that the reddish areas on non-consecrated wafers resulted from fungal growth. In addition, these data showed that a general examination under ultraviolet light can prove useful in the initial characterization of enigmatic reddish areas and may also provide further diagnostic information for various past events.
Figure 5: Ultraviolet fluorescence of whole blood and reddish growths on non-consecrated wafers. (A) Blood was applied to non-consecrated wafers and examined together with reddish growths on wafers under visible light (VL) and ultraviolet light (UV). Unlike whole blood, reddish growths exhibit red/orange fluorescence under UV. (B) Reddish portions of wafers were examined under visible light (VL) and ultraviolet light (UV); the sample on the right-hand side was viewed through a stereomicroscope.
Figure 6: Microscopic examination of reddish growths on non-consecrated wafers. Samples were examined under 100x (A) and 400x (B). Hyphae are indicated in (A) with arrows. Structures in (B) were identified as conidia and chlamydospores, similar to those seen with Epicoccum nigrum.
Figure 7: Microscopic examination of reddish growths treated with fungal stain. Samples were treated with the fungal stain lactophenol cotton blue and examined under 100x (A) or 400x (B). Hyphae and a wafer strand are indicated by black and white arrows, respectively.
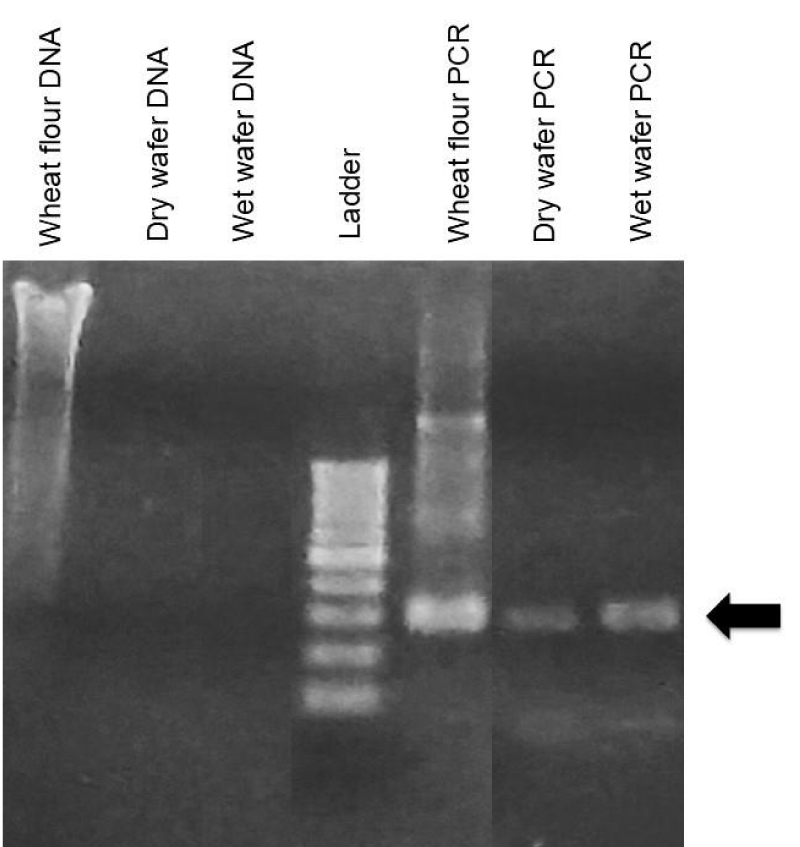
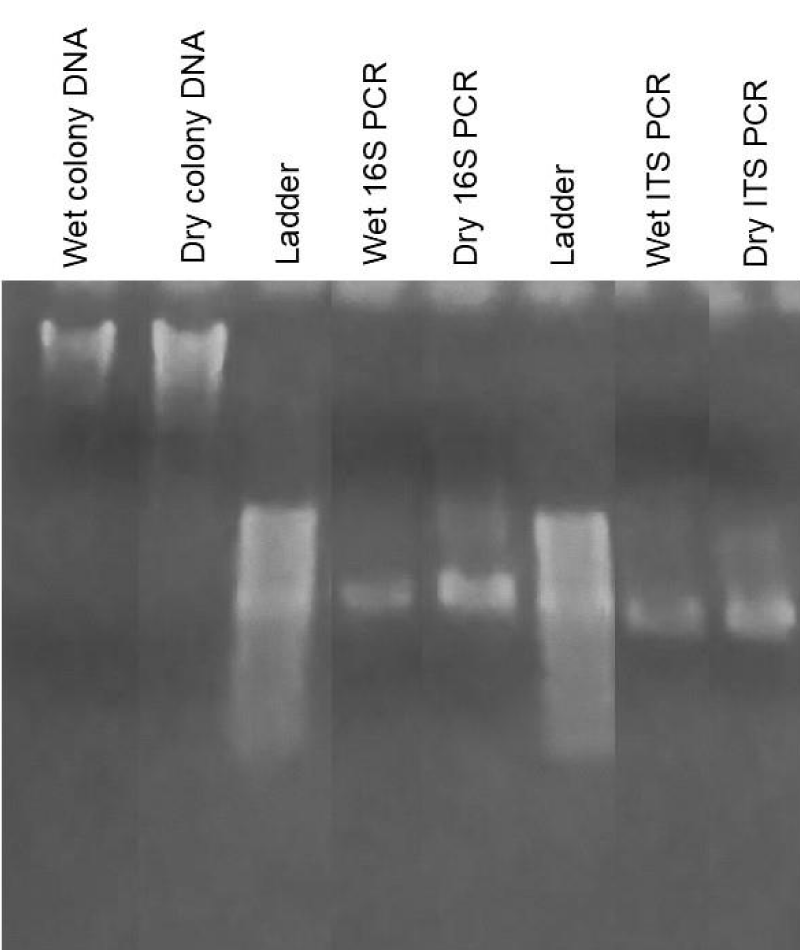
In our next set of studies, we wished to examine what types of non-human DNA might exist in unconsecrated wafers. In previous miracle cases, the non-amplification of DNA by human-specific primers was suggested to represent a type of divine property. We reasoned that three potential sources of non-human DNA might exist under such conditions: DNA originating from the wheat wafer itself, and DNA from bacteria and fungi. Tilley has reported that wheat DNA can survive baking conditions in studies of oven-cooked wheat bread, which is sufficiently stable to be amplified by PCR using wheat-specific primers [19]. As shown in Figure 8, whole DNA was very fragmented in unconsecrated wafers but was sufficiently intact to be amplified using PCR, giving DNA product of the expected size (Figure 8, arrow), [19]. This sequence aligned with Triticum aestivum, wheat bread, Genbank accession number MG560142, with 93.07% identity, with many of the mismatches occurring over bases with lower confidence in the samples produced in this project. We assume the low quality of the sequencing product was due to DNA degradation during the processing and baking of the wafers. Whole DNA was also purified from cultured wafers with reddish growths, which showed a much stronger signal compared to non-treated wafers (Figures 8,9). PCR amplification was performed using 18S and ITS-specific primers [28,29], (Figure 9), followed by Sanger sequencing to aid in identification of bacterial and fungal DNA, respectively. None of the bacterial products produced sequences that were distinctly interpretable; this is assumed to be because any bacteria found in the samples were of mixed types. Fungal primer samples produced a consensus sequence that was 313 bp long, which matched several fungal sequences in Genbank with 99.35% identity, specifically Ascochyta sp., Chaetomium globosum, Epicoccum italicum, Epicoccum nigrum, Epicoccum sorghinum, Fusarium equiseti, and Pleosporales sp. Visual identification of macroscopic and microscopic properties by various fungal specialists, together with DNA sequencing studies, indicated the presence of Epicoccum sp. Definitive sequencing to identify the specific Eppicoccum species present in cultured wafers was beyond the scope of the current study, although Bell and Karuso noted in 2003 that Epicoccum nigrum produces a pigment that fluoresces with similar colors under ultraviolet light as we observed [30], making this a likely candidate. Taken together, these data indicate that multiple sources of non-human DNA could be isolated from cultured wafers, including those from plants, bacteria, and fungi. Importantly, these results identified Epicoccum fungus as the most likely cause for the observed reddish growths in our study.
Figure 8: DNA analysis of non-consecrated, non-treated wheat wafers. Whole DNA was extracted from non-consecrated wheat wafers and analyzed by gel electrophoresis. Whole DNA from wheat flour is also shown for comparison. DNA was amplified by using wheat-specific primers which gave a major band of the expected size (arrow). Sanger sequencing gave a 93% identity match for the expected wheat product.
Figure 9: DNA analysis of reddish growths on non-consecrated wheat wafers. Whole DNA was extracted from reddish growth areas on non-consecrated wheat wafers and analyzed by gel electrophoresis. Whole DNA from wet or dried reddish growths is shown, together with amplification using 16S and ITS primers, specific for bacteria and fungi, respectively. Major bands of the expected size were obtained, and sequencing results showed a mixed array of bacteria and a limited number of fungal candidates.
While much attention has been placed on the evaluation of consecrated hosts suggested to exhibit miraculous properties of visible blood, we show here for the first time that ordinary, non-consecrated wafers show similar appearances when treated in a like manner. As demonstrated, while a small percentage of unconsecrated wafers may have a similar physical appearance as bleeding bread, basic scientific tests can readily distinguish between the two, including a simple evaluation with an ultraviolet flashlight. While a few cases have been described in which consecrated hosts were not immersed in water prior to the appearance of a miracle, it is reasonable to assume that contamination and growth could also occur through handling, high humidity, etc. Moreover, multiple strains of bacteria and fungi are known to produce reddish pigments, which are likely to differ depending on the specific location and environmental conditions. Thus, it is probable that variation exists in cases where microorganisms are believed to be the reason for the occurrence of certain miracle events. Each instance would need to be examined individually in as thorough a scientific manner as possible (see below). In our case, the causative agent was identified as a fungal growth, Epicoccum sp., most likely Epicoccum nigrum, that exists worldwide.
Similar to what has been described for various miracle hosts, we found that the water in which the wafers were placed did not become tainted over time. If real blood were being issued forth, this would be a relatively odd property as blood should be readily solubilized. Indeed, as shown in our studies, fresh blood or freshly dried blood on wafers dissolved in water relatively quickly as expected. We found that only about 15% of non-consecrated wafers cultured in water developed reddish, blood-like areas, supporting the idea that reports of miracles are relatively infrequent and do not occur every time abandoned hosts are disposed of according to church protocol. Although our studies did not implicate Serratia marcenes as a causative agent, it is understandable how this common infectious agent of bread substances could produce the appearance of a bleeding host. Several basic tests were shown to easily distinguish Serratia growth from genuine blood, including Wright’s stain and spectroscopy. We attempted to perform similar tests on reddish growths from fungus but found that they were not readily solubilized in a variety of detergents or solvents.
One of the most enunciated pieces of evidence for the validity of Eucharistic miracles is their shared blood type, AB, which has also been reported for the Shroud of Turin. Although AB typing experiments were beyond the scope of the current study, it should be noted that bacteria also express AB antigens [15,31], providing a practical explanation for similar results among a wide variety of cases. Indeed, both blood fibers of the Shroud and various Eucharistic miracles have been reported to be substantially contaminated with both bacteria and fungi [4,15,32], similar to our findings with non-consecrated wafers.
Molecular biology studies of miracle wafers have been attempted, particularly short tandem repeat (STR) analysis, which has failed to produce any results and thereby attributed to an enigmatic and “divine” nature of the DNA involved [4,18]. As shown in the current report, the prevalence of DNA from other species (plant, bacterial, fungal) in non-consecrated wafers offers a plausible alternative to the findings of previous Eucharistic miracle investigations. Additional DNA testing using sequences that are both specific to humans and vary among individuals would be required to strengthen the claim that human DNA present in multiple Eucharistic miracles share a common origin (see below).
Scientific validation that various Eucharistic miracles share a common origin requires at a minimum the demonstration that specific identifiers are mutually present. The human leucocyte transplantation antigens (HLA) are the most polymorphic protein and genetic systems present in man [33,34] and offer the distinctive opportunity to validate that tissue proteins and/or DNA truly originate from the same source. In the case that DNA from miracle wafers is problematic due to contamination by multiple individuals, HLA protein analysis via immunohistochemistry methods could provide an alternative solution.
One of the most intriguing properties of Eucharistic miracle events is the presentation of histological sections corresponding to cardiac tissue [2,4]. If the conversion is truly valid, the HLA expression should accompany this transformation. Multiplex immunohistochemistry methods have shown that simultaneous evaluation of multiple biomarkers in a single tissue section is possible, which could prove helpful in such studies [35,36]. Evaluation of HLA markers also provides a vital cross-check for the submission of fraudulent samples: an identical profile should be obtained in all instances, worldwide, if the claim of a single origin is legitimate. This is very important.
Finally, the authors wish to state that the purpose of this article is not to suggest that all Eucharistic miracles should be viewed as fallacious; rather, the aim is to emphasize that solid scientific practice should be used in the evaluation of such occurrences so that a true assessment may take place. Faith and science need not contradict one another; however, one of the major issues with the subject of Eucharistic miracles is that no standard scientific protocol exists for their examination. To this end, a suggested minimum procedure is described below to augment any future investigation and serve as a starting point to standardize the scientific evaluation of such occurrences. It is also foreseen that additional criteria may be included to aid in the improved reproducibility and reliability of results.
Initial documentation of the event and all people involved
A detailed description of the event, including photographs at all stages of the initial occurrence should be provided. If it is decided that an investigation should be performed, it is recommended that the substance be placed inside a container bearing the seal of the appropriate church authority. If DNA studies are a future consideration, swab samples should be taken from anyone in contact/proximity to the substance at any stage of the investigation. If necessary, such samples could be evaluated in the future to exclude potential contamination, especially in reference to STR or mt DNA analysis. Handling conditions and use of PPE should be taken into consideration as early as possible. At each stage of the examination, the chain of custody should be meticulously documented using both written and photographic formats.
Differential staining, spectroscopy and histological studies
Simple staining for blood and various microorganisms can provide insight into the potential contamination level of wafers suspected to have undergone a miracle event and may prove useful in the initial characterization of what may or may not be present. Spectroscopy techniques, which have not been used in prior evaluations, may help to provide a rapid, predictable assessment if blood is truly present. One of the most intriguing aspects of Eucharistic miracles is the suggestion that the substance of a wheat wafer transforms into cardiac tissue, particularly that from an individual who has suffered physiological trauma. An obvious concern is the fact that only a limited number of individuals have been involved in sample extraction. It would be helpful if additional scientific “outsiders” were present during sample removal and where possible, multiple samples should be taken for separate delivery and examination by various labs. One of the potential shortfalls of previous studies has been the sole reliance on targeted specialists and the lack of evaluation of specific markers for cell types/tissues of interest. Scientists from multiple disciplines need to be involved in the visual inspection of the samples, including general pathologists, cardiologists, microbiologists, mycologists, and plant pathologists. In today’s digital age, many opinions can be solicited electronically. Additionally, experimental samples need to be presented together with various control slides in a blind fashion to solicit as objective an opinion as possible. The inclusion of samples from non-consecrated wheat wafers subject to similar conditions that result in the appearance of a “bleeding” host should also be considered.
In histological studies, it is essential that the identification of various blood cell types (neutrophils, eosinophils, etc.) not be made via morphological assessment alone. Cell-specific markers should be utilized to help verify their identity. Modern multiplex immunohistochemistry methods may prove very useful in this regard (see above). Lastly, it is important to emphasize that in any type of serological study, specificity controls always need to be included and presented to verify that nonspecific binding is limited under such conditions. In many prior presentations of Eucharistic miracle findings, these have been noticeably absent and are essential to increase the scientific rigor of the results.
Blood characterization, ABO and HLA typing
A simple evaluation using ultraviolet light might be informative in the initial characterization of samples thought to contain real blood. Studies involving ABO blood typing should strongly consider the potential pitfalls involved with serological methods recognizing shared antigens among species. This is particularly important when both macroscopic and microscopic examinations indicate the presence of microorganisms in the sample. ABO typing of contaminated objects using antibody-based methods is relatively futile; alternative experimental approaches should be considered that do not rely on serological methods which detect common antigens. Although surface AB antigens are shared between humans and bacteria, Yamamoto demonstrated in 2004 that the enzymes that add such structures (glycosyltransferases) are distinct between species [31]. PCR-based molecular techniques probing for human vs bacterial GT offer a potential way to circumvent such issues, providing a more definite and accurate evaluation of ABO origin [15]. If anti-ABO typing is done using antibody-based methods, specificity controls using both unrelated antibodies and irrelevant blood types should be included to further verify the results. Serological testing using anti-glycophorin A is very valuable as it recognizes a marker that is both red blood cell and human-specific. However, it should be emphasized that all pertinent data involving specificity controls need to be included in the presentation of the results. A positive antibody binding result is meaningless without explicit demonstration that the recognition is specific, especially in cases where potentially extraordinary events are involved. Ultimately, it would need to be demonstrated that unique specific (polymorphic) identifiers are present to validate that samples originate from the same source. HLA analysis provides a distinctive approach to authenticate the genuine shared origin of these occurrences, which could be evaluated at the protein and/or DNA level.
Molecular biology studies
In future investigations, it is suggested that contact with the substance should be minimized, particularly with individuals not equipped with PPE (including masks). While it is acknowledged this is not part of the normal liturgical procedure, it is an important detail in the scientific investigation that may follow. DNA samples from all individuals in contact with the sample at any stage of the investigation should be collected and stored for future reference, if necessary. In all previous studies, DNA analysis was restricted exclusively to human-specific genes; however, the possibility of DNA being present from non-human sources (bacteria, fungus, plant) needs to also be considered. The documented use of spike controls to rule out the presence of PCR inhibitors in the sample should be incorporated as needed. From a scientific standpoint, it is important that all-natural possibilities be exhaustively evaluated before suggestions that the sample is of a divine nature are put forth. Molecular biology techniques provide the opportunity to establish the presence or absence of a unique, specific identifier, such as STR, mitochondrial DNA genes, or HLA, a necessary and important issue for supporting the claim that such events truly originate from a shared source.
Presentation of scientific results and conclusions to the public
The normal course of action in any scientific investigation is to write up the results for submission to a scientific journal so that the findings may be critically and constructively evaluated. Scientific transparency is important for the establishment of the belief that such extraordinary events might be possible. Premature reporting by press release of incomplete conclusions should be avoided. Relatedly, liturgical representatives should be particularly diligent in fact-checking the scientific claims that often surround such events and update any current websites and publications regularly.
In summary, the current report has evaluated the results from various Eucharistic miracles with particular attention to the methodology used in the analysis. Additionally, evidence was provided that the appearance of a bleeding host can occur by placing a non-consecrated wafer under similar conditions as described for many of these events. Distinctions between ensuant reddish areas and genuine blood on experimental wafers were noted, and ultraviolet light was shown to be a useful discriminator. Our studies indicated the presence of a particular fungus being responsible for reddish growths on wafers, in this case, Epicoccum sp. Lastly, suggestions toward establishing a minimal protocol of scientific examination were put forward to help standardize the investigation of possible miracle occurrences in the future.
Thank you to the many investigators who provided helpful discussion and assistance, especially Drs. June Kwon Chung (NIH), Rob Dunn (NC State University), David Michael Geiser (Penn State University), Allan Gillen (Liberty University), David Golden (University of Tennessee), and Mike Tilley (University of Kansas).
The expert technical assistance of Veronica Brown and Andrea Trent (University of Tennessee Genomics Core) is also recognized and appreciated.
- Spitzer R. Contemporary Scientifically Validated Miracles Associated with Blessed Mary, Saints and the Holy Eucharist. Magis Center; 2020.
- Hemler S, Granger L. The science of Eucharistic miracles. Arlington Catholic Herald. 2022.
- Real Presence Eucharistic Education and Adoration Association, Inc. The Eucharistic Miracles of the World. Eternal Life; 2016.
- Serafini F. A Cardiologist Examines Jesus. Sophia Institute Press; 2021.
- Gomez RC. Mas alla de la Razon. Grupo Internacional Para La Paz Centro Internacional De Estudios Humanos; 2009.
- Becker R. Eucharistic miracles and the divine blood type. National Catholic Register. 2018. Available from: https://www.ncregister.com/blog/eucharistic-miracles-and-the-divine-blood-type
- Senz P. Doubt the Real Presence? Try a blood miracle. Catholic Answers Magazine. 2021. Available from: https://www.catholic.com/magazine/online-edition/doubt-the-real-presence-try-a-blood-miracle
- Manning P. A mathematical case for the Real Presence. Catholic Answers Magazine. 2023. Available from: https://www.catholic.com/magazine/online-edition
- Armstrong PM. Eucharistic miracle? ‘Bleeding host’ phenomenon reported in dioceses worldwide. National Catholic Register. 2015. Available from: https://www.ncregister.com
- Gillen AL. Serratia marcescens: The miracle bacillus. 2012. Available from: https://www.catholic.com/serratiamarcescens
- Bennett JW, Bentley R. Seeing red: the story of prodigiosin. Adv Appl Microbiol. 2000;47:1-32. Available from: https://doi.org/10.1016/s0065-2164(00)47000-0
- Celedón RS, Díaz LB. Natural pigments of bacterial origin and their possible biomedical applications. Microorganisms. 2021;9(4):739. Available from: https://doi.org/10.3390/microorganisms9040739
- Afroz Toma M, Rahman MH, Rahman MS, Arif M, Nazir KHMNH, Dufossé L. Fungal pigments: carotenoids, riboflavin, and polyketides with diverse applications. J Fungi (Basel). 2023;9(4):454. Available from: https://doi.org/10.3390/jof9040454
- Linoli O. Histological, immunological and biochemical studies on the flesh and blood of the Eucharistic Miracle of Lanciano (8th century). Quad Sclavo Diagn. 1971;3:661-74. Available from: https://pubmed.ncbi.nlm.nih.gov/4950729/
- Kearse KP. The relics of Jesus and Eucharistic miracles: scientific analysis of shared AB blood type. Forensic Sci Med Pathol. 2024. Available from: https://doi.org/10.1007/s12024-024-00915-3.
- Weiring M. Eucharistic miracle science may bolster, but should not detract from faith, say experts. Catholic Review. 2023. Available from: https://catholicreview.org/eucharistic-miracle-science-may-bolster-but-should-not-distract-from-faith-say-experts/
- Trasancos S, Elliot G. Behold It Is I. TAN Books; 2021.
- Tesoriero R, Han L. Unseen new evidence: the origin of life under the microscope. Ron Tesoriero; 2013.
- Tilley M. PCR amplification of wheat sequences from DNA extracted during milling and baking. Cereal Chemistry. 2004;81:44-47. Available from: https://doi.org/10.1094/CCHEM.2004.81.1.44
- Alberti MO, Drake TA, Song L. The pH of chemistry assays plays an important role in monoclonal immunoglobulin interferences. Pract Lab Med. 2015;3:8-16. Available from: https://doi.org/10.1016/j.plabm.2015.09.001
- Sukumaran A, Thomas T, Thomas R, Thomas RE, Paul JK, Vasudevan DM. Development and troubleshooting in lateral flow immunochromatography assays. Indian J Clin Biochem. 2020;36:1-5. Available from: http://dx.doi.org/10.1007/s12291-020-00887-5
- Virgolini I, Zelger B, Zelger B, Kenner L. Reality or fiction of the “Real Presence” of Jesus Christ in the Holy Eucharist? Ann Case Rep. 2023;8:1289. Available from: https://doi.org/10.29011/2574-7754.101289
- Rodak BF, Fritsma GA, Doig K. Hematology: Clinical Principles and Applications. Saunders; St. Louis, MO: 2007;816. Available from: https://books.google.co.in/books/about/Hematology.html?id=6sfacydDNsUC
- Hanson EK, Ballantyne J. A blue spectral shift of the hemoglobin soret band correlates with the age (time since deposition) of dried bloodstains. PLoS One. 2010;5(9):e12830. Available from: https://doi.org/10.1371/journal.pone.0012830
- Maciel C, Fujita A, Gueroni DI, Ramos AD, Capurro ML, Sá-Nunes A. Evans blue as a simple method to discriminate mosquitoes' feeding choice on small laboratory animals. PLoS One. 2014;9(10):e110551. Available from: https://doi.org/10.1371/journal.pone.0110551
- The Eucharistic miracle of Sokolka. Aleteia — Catholic Spirituality, Lifestyle, World News, and Culture. 2017 Sep 23.
- Kearse KP. Environmental influence on blood serum detection using ultraviolet 365. J Forensic Sci Res. 2021;5:030-036. Available from: https://doi.org/10.29328/journal.jfsr.1001024
- Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41(1):e1. Available from: https://doi.org/10.1093/nar/gks808
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols. San Diego: Academic Press; 1990;315-322. Available from: http://dx.doi.org/10.1016/B978-0-12-372180-8.50042-1
- Bell PJ, Karuso P. Epicocconone, a novel fluorescent compound from the fungus Epicoccum nigrum. J Am Chem Soc. 2003;125(31):9304-5. Available from: https://doi.org/10.1021/ja035496+
- Yamamoto F. Review: ABO blood group system—ABH oligosaccharide antigens, anti-A and anti-B, A and B glycosyltransferases, and ABO genes. Immunohematology. 2004;20:3-22. Available from: https://pubmed.ncbi.nlm.nih.gov/15373665/
- Garza-Valdes L. The DNA of God? Doubleday, Holy Shroud, USA; 1999.
- Dunn PPJ. Human leucocyte antigen typing: techniques and technology, a critical appraisal. Int J Immunogenet. 2011;38:463-73. Available from: https://doi.org/10.1111/j.1744-313x.2011.01040.x
- Hurley CK. Naming HLA diversity: A review of HLA nomenclature. Hum Immunol. 2021 Jul;82(7):457-465. Available from: https://doi.org/10.1016/j.humimm.2020.03.005
- Tsujikawa T, Kumar S, Borkar RN, Azimi V, Thibault G, Chang YH, et al. Qualitative multiplex immunohistochemistry reveals myeloid-inflamed tumor-immune complexity associated with poor prognosis. Cell Rep. 2017;19:203-17. Available from: https://doi.org/10.1016/j.celrep.2017.03.037
- Radtke AJ, Chu CJ, Yaniv Z, Yao L, Marr J, Beuschel RT, et al. IBEX: an iterative immunolabeling and chemical bleaching method for high-content imaging of diverse tissues. Nat Protoc. 2022;17(2):378-401. Available from: https://doi.org/10.1038/s41596-021-00644-9