More Information
Submitted: September 23, 2024 | Approved: September 26, 2024 | Published: September 27, 2024
How to cite this article: Aggarwal B. The Gut-Brain Axis: Exploring the Bidirectional Communication Between the Gut Microbiome and the Brain. J Forensic Sci Res. 2024; 8(1): 047-057. Available from: https://dx.doi.org/10.29328/journal.jfsr.1001064
DOI: 10.29328/journal.jfsr.1001064
Copyright License: © 2024 Aggarwal B. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Gut-Brain Axis, Gut Microbiome, Enteric Nervous System, Gut Hormones, Neurological Disorder
The Gut-Brain Axis: Exploring the Bidirectional Communication Between the Gut Microbiome and the Brain
Bhoomi Aggarwal*
Department of Forensic Science Vivekananda Global University Jaipur, Rajasthan, India
*Address for Correspondence: Bhoomi Aggarwal, Department of Forensic Science Vivekananda Global University Jaipur, Rajasthan, India, Email: [email protected]
The gut microbiome is a complex network of interactions between the brain and the gastrointestinal tract, playing a pivotal role in human health and disease. The microbiota-gut-brain axis (GBA) serves as a crucial connector between the brain’s emotional and cognitive centers and the peripheral intestinal functions, emphasizing the profound impact of gut health on overall well-being. The GBA is characterized by a symbiotic relationship between the gut and the brain, regulating the expression of inflammatory cytokines and neurotransmitters. The MGBA is also regulated by microbial metabolites, such as short-chain fatty acids (SCFAs) and fatty acid derivatives. This paper focuses on the importance of the GBA in regulating gut health and the potential for targeted therapeutic interventions to improve health outcomes. The implications of this research are vast, suggesting that future strategies aimed at modulating the gut biome may offer promising avenues for the development of personalized medicine and dietary interventions.
The intricate communication network known as the Gut-Brain Axis (GBA) serves as a pivotal connector between the brain’s emotional and cognitive centers and the peripheral intestinal functions, emphasizing the profound impact of gut health on overall well-being [1]. This axis encompasses a wide array of systems including the Central Nervous System (CNS), Autonomic Nervous System (ANS), Enteric Nervous System (ENS), and Hypothalamic Pituitary Adrenal (HPA) axis, all of which play crucial roles in regulating the complex interactions between the gut microbiome and brain health [1-3]. The significance of the gut-brain axis extends beyond mere physiological processes, influencing various aspects of human health and disease, ranging from mood and cognitive functions to gut health and metabolism, thereby highlighting the deep interconnectedness of the gut microbiome with neurological and psychological health [4-6]. Recent findings underscore the profound influence of the gut microbiome on the gut-brain connection, revealing that alterations in the composition of gut microbiota can affect not only gut health but also have far-reaching effects on neurotransmitters and the development of conditions such as autism [1,4]. The dialogue between the gut and brain is mediated through multiple pathways including neuroendocrine and metabolic routes, underscoring the gut-brain axis as a fundamental aspect of both health and disease. Through its ability to regulate cells in nearby and distant organs, such as the brain, the gut microbiota that lives in the Gastrointestinal (GI) tract has a significant impact on the host’s overall health. In the Gut-Brain Axis (GBA), bidirectional transmission takes place as a two-way communication channel between the host’s neurological system and gut. The immune system, hormones, and brain networks can all transmit this information, supporting the intestinal flora. In the GBA, bidirectional communication controls the innate and adaptive immune systems, preserves a mutualistic relationship with the host, and governs brain dysfunction mechanistically [4,7]. This axis involves multiple pathways, including the immune system, the Hypothalami-Pituitary-Adrenal (HPA) axis, the endocrine system, the autonomic and enteric nerve systems, and the microbiota and its metabolites. To communicate with host cells, including intestinal epithelial cells (IECs) and immune cells, a healthy gut microbiota produces microbial metabolites and neurotransmitters. Many immune-associated neurological illnesses, such as emotional dysregulation, neurodegeneration, and developmental abnormalities, have been tied to changes in the gut microbiota and microbial metabolite synthesis [2,3,4]. The brain is the organ that controls and is accountable for all of a person’s conduct. It is made up of numerous distinct populations of both neuronal and non-neuronal cells connected by extraordinarily complex structural networks. The Digestive Tract (GI) is the habitat for more than 98% of the bacteria in our bodies [8]. The term “gut microbiota” refers to the particular microorganisms that are present and reside in the gut. This sets the stage for a deeper exploration into how disruptions in the gut-brain axis can manifest in various disorders, and how understanding this complex communication network opens new avenues for therapeutic interventions targeting gut health and neurological diseases [5,9,10].
Understanding the microbiota-gut-brain-axis
The Microbiota-Gut-Brain Axis (MGBA) is a sophisticated network of interactions involving the gut microbiota, the gastrointestinal tract, and the brain, playing a pivotal role in human health and disease [1]. This section delves into the various components and functionalities of the MGBA, highlighting its significance in maintaining physiological homeostasis and its implications in various disorders.
Components of the gut-brain axis
The gut microbiome consists of bacteria, archaea, viruses, and eukaryotic microbes that colonize the digestive tract [11]. The gut microbiota, which comprises approximately 100–150 times more genes than the human genome, is found in the human intestines and includes approximately 1,000 species and 7,000 types of bacteria, gram-positive or gram-negative Firmicutes (including the species Lactobacillus, Eubacterium, and Clostridium), and gram-negative Bacteroidetes form the majority of the bacteria (containing Bacteroides and Prevotella) [11,12]. The gut-brain axis includes several critical systems such as the central nervous system (CNS), autonomic nervous system (ANS), enteric nervous system (ENS), and the hypothalamic-pituitary-adrenal (HPA) axis [1,3]. These components interact complexly with the gut microbiota to regulate bodily functions ranging from stress response to gastrointestinal motility [13].
- Central Nervous System (CNS): Communicates with intestinal targets through autonomic pathways, influencing gut functions like motility and mucus secretion.
- Autonomic Nervous System (ANS): Facilitates direct neural communication between the gut and the brain.
- Enteric Nervous System (ENS): Often referred to as the "second brain," it governs the function of the gastrointestinal system and communicates with the CNS [2,11].
- Hypothalamic-Pituitary-Adrenal (HPA) axis: Activates during stress, releasing cortisol, which affects various bodily functions including gut permeability.
Microbial influence and dysbiosis
The human gut hosts a complex community of microorganisms that significantly impact health and disease. Dysbiosis, or the imbalance of these gut microbes, has been linked to several central nervous disorders and functional gastrointestinal disorders such as Irritable Bowel Syndrome (IBS) [3,14].
- Dominant microbial phyla: The gut microbiota predominantly consists of the phyla Firmicutes and Bacteroidetes, which play crucial roles in metabolic processes and maintaining gut health.
- Impact of dysbiosis: Altered microbial compositions have been associated with conditions like autism, anxiety, depression, and IBS, highlighting the critical role of balanced microbiota in brain and gut health [15].
Signaling pathways in the MGBA
The communication within the MGBA involves various signaling mechanisms including neural, endocrine, and immune pathways, which are influenced by microbial metabolites such as Short-Chain Fatty Acids (SCFAs) and tryptophan metabolites [11,14].
Neuroimmune and neuroendocrine interactions: These pathways facilitate the bottom-up modulation of the CNS, primarily involving the vagus nerve, impacting emotional and physical health [16].
Microbial metabolites: Compounds like SCFAs and secondary bile acids play significant roles in signaling within the MGBA, affecting everything from satiety to stress response.
Therapeutic implications
Understanding the microbiota-gut-brain axis opens new avenues for therapeutic interventions targeting various CNS disorders. By modulating the gut microbiota, it may be possible to alleviate symptoms of diseases like IBS and even influence psychological well-being [4,17].
- Potential therapies: Targeting microbial-derived metabolites and improving gut barrier functions are promising strategies for managing CNS disorders and enhancing overall health.
- Role of diet: Dietary interventions can alter the gut microbiota composition rapidly, influencing the MGBA and potentially mitigating disease symptoms.
This exploration of the microbiota-gut-brain axis underscores its complexity and the significant impact of gut health on overall well-being and disease. Understanding these intricate connections helps in devising targeted interventions that could revolutionize treatments for numerous health issues linked to the gut-brain [15,18,19].
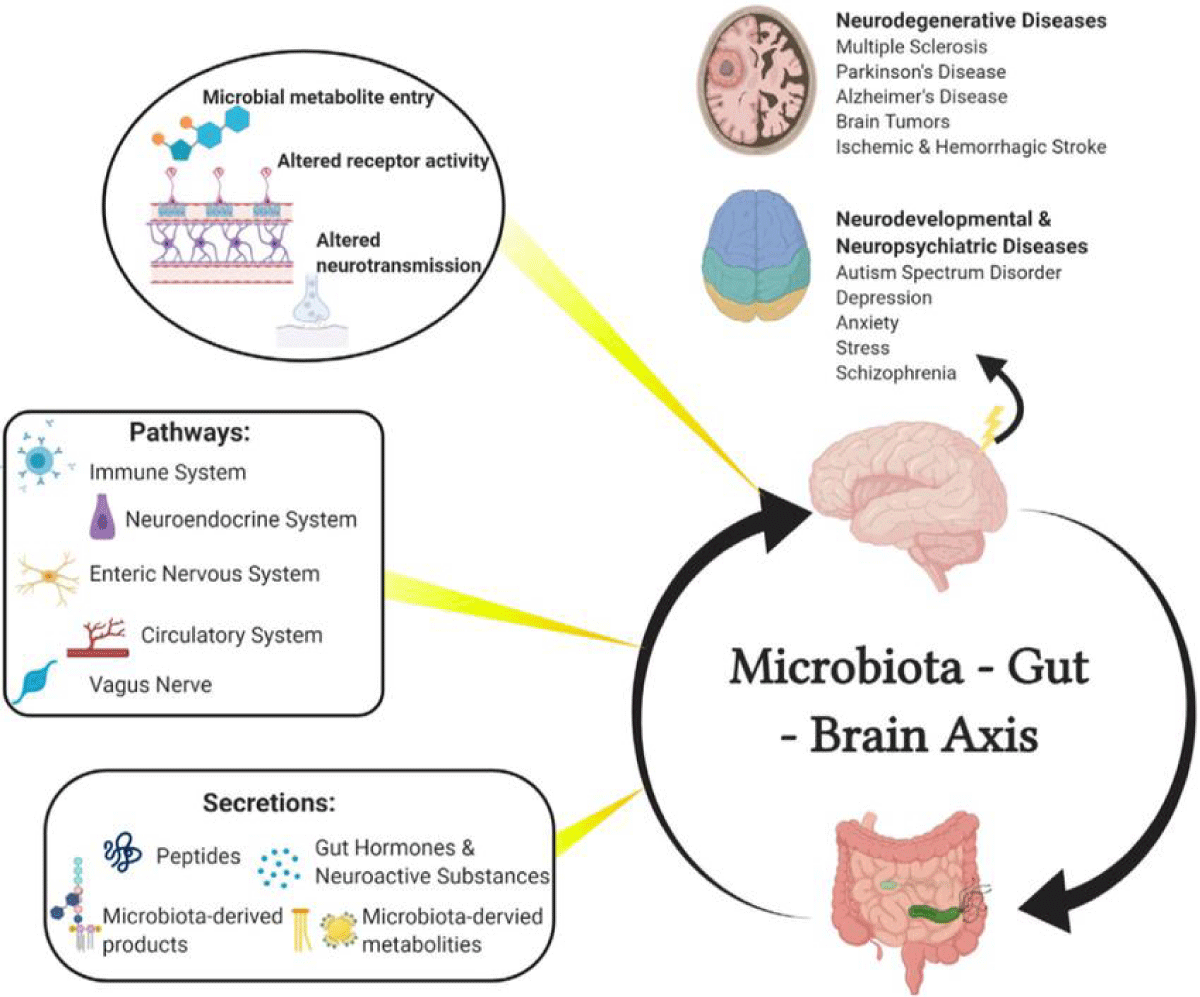
Numerous routes, including the immunological system, neuroendocrine system, enteric nervous system (ENS), circulatory system, and vagus nerve, mediate the bidirectional connection between the brain and gut bacteria. Numerous neuroactive chemicals, including peptides, gut hormones, and metabolites and products of microbes, are found along the paths of these circuits. Once metabolites are within the brain, they can affect neurodevelopment and neurodegeneration in a variety of disorders, including multiple sclerosis, stroke, CNS cancers, Parkinson’s disease, Alzheimer’s disease, autism spectrum disorder, depression, anxiety, stress, and schizophrenia [4,18] (Figure 1).
Figure 1: Diagrammatic representation of the microbiota-gut-brain axis [18].
How the gut microbiota affects the brain
Direct neural, immunological, and endocrine routes are only a few of the chemical signaling pathways that the CNS and ENS use to interact with one another. The gastrointestinal, neurological, and microbial systems of animals depend on the gut-brain axis, a web of linkages comprising several biological systems that enable two-way communication between gut bacteria and the brain [1], The gut microbiota influences the brain not only through the nervous system but also through the endocrine, immunological, and metabolic systems (the gut-brain neuroanatomical route). Because the gut microbiota can be purposefully altered and used as an independent variable, the role of bacteria in the gut microbiota-brain axis is being heavily stressed. For example, microbes can modify the expression of neurotrophic factors and N-methyl D-aspartate (NMDA) receptor subunits in the hippocampus, which can have an impact on how the nervous system grows, matures, ages, and maintains homeostasis [7,14,20]. The immune system, chemical transmitters, neural routes, endocrine pathways, and other biological networks are the primary mechanisms via which the microbiota can impact the growth and operation of the nervous system.
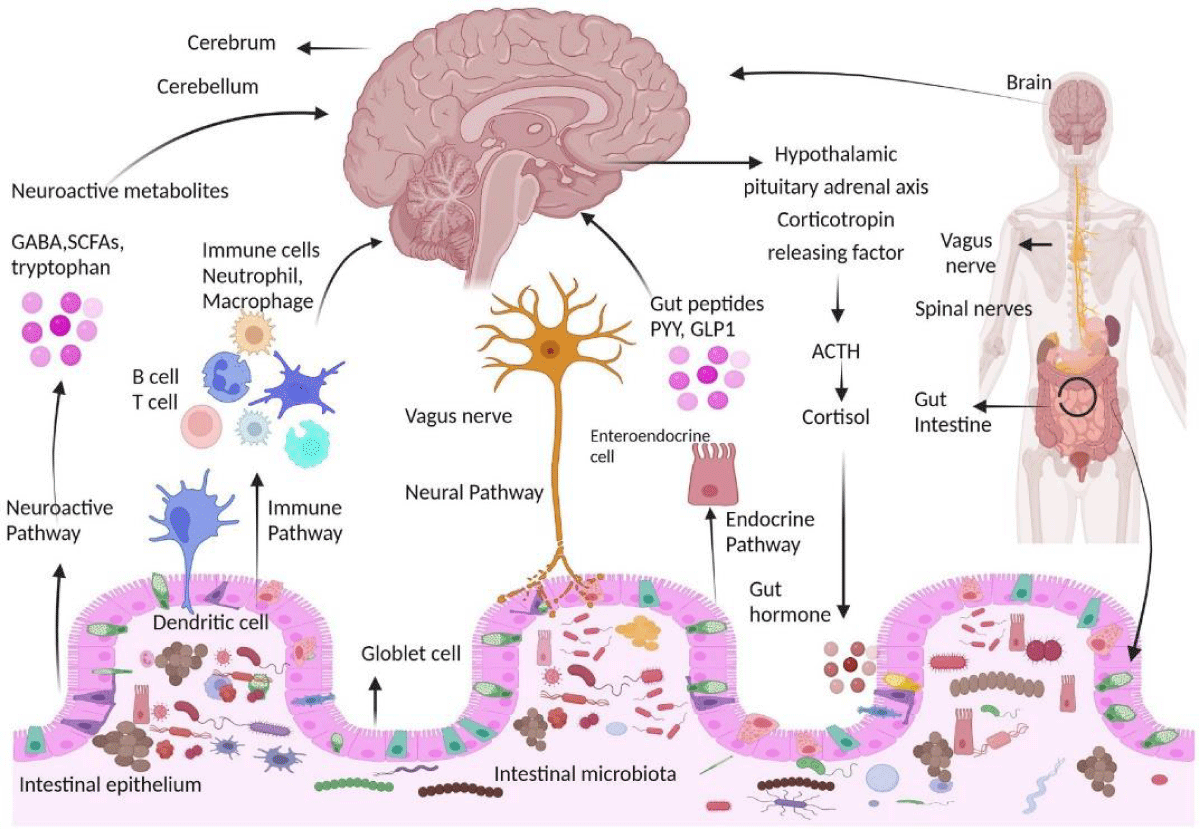
Communication pathways between the brain and gut microbiota. The interaction between the central nervous system (CNS) and gut microorganisms is mediated via several direct and indirect gut-brain axis mechanisms [1,21] (Figure 2).
Figure 2: Gut microbiota affects the brain [1].
Microbiota and neurotransmitters
Through “direct” and “indirect” chemical interactions with the neurological system, gut bacteria can influence behavior and help control body processes in their animal host. Some of the neuroactive chemicals are synthesized by microorganisms, and they can also induce the host to produce additional metabolites and neurotransmitters that control gut-brain transmission [1,22]. The proper maturation, activation, and development of microglia—the brain’s innate immune cells—requires the microbiota. Microglial form and function are restored in Germ-Free (GF) mice when bacterial-derived short-chain fatty acids (SCFAs) are administered. This suggests that signals from microbial metabolism influence immunological conditioning by microglia. Chemicals are produced by microbes communicating with the brain [1,23]. The gut microbiota produces neurotransmitters that affect brain γ-aminobutyric acid (GABA) differently, including dopamine, serotonin, norepinephrine, glycerine, and gamma-aminobutyric acid. Disorders like AD, PD, autistic spectrum disorder, anxiety disorders, and depressive disorders can result from imbalances in these neurotransmitters.
For example, GABA can be produced by Lactobacillus and Bifidobacterium; noradrenaline can be produced by Bacillus, Saccharomyces, and Escherichia; serotonin can be produced by Bacillus, Candida, Escherichia, and Enterococcus species; bacteria can produce dopamine; and Lactobacillus can produce acetylcholine [22]. Bifidobacterium infantis also raises blood plasma tryptophan levels, which impacts central serotonin transmission. The lipids produced by intestinal microorganisms during the fermentation of dietary fiber are known as SCFAs, and they are a form of direct signaling that can affect the CNS’s neuroplasticity, epigenetics, and immune system. Butyrate, propionate, and acetate are essential metabolic byproducts of gut microbial activity that have an impact on the brain, energy balance, and metabolism [10,23,24]. Furthermore, SCFAs function as endogenous ligands for orphan G protein-coupled receptors (GPCRs), and via inhibiting histone deacetylases, intracellular SCFAs control gene expression. Furthermore, SCFAs have an impact on hormone control and inflammation through their interaction with vagal afferents [25]. The hypothesis that SCFAs can be important players in GMB communication is supported by the interactions of SCFAs with specific cellular systems and gut-brain signaling pathways. The microbial modulation of the neuroendocrine system is one way that the microbiota indirectly communicates with the nervous system and behavior [26,27]. By altering the endocrine signals generated by enteroendocrine cells (EECs) in the gut epithelium, which involve the synthesis of the hormone glucagon-like peptide 1 (GLP1), gut bacteria can influence their host’s appetite and eating habits. In addition to producing neurotransmitters on their own, the gut microbiota of animals can also encourage the production of these molecules in their hosts. For instance, it is known that some bacteria, such as Escherichia spp., Bacteroides, Bifidobacterium, and its species, produce the neurotransmitter GABA. In animal mouse model systems, it has been shown that bacteria are necessary for the synthesis of the neurotransmitter serotonin 5-hydroxytryptamine (5-HT) [1,5,28].
Microbial influence on glial functions
Glial cells and neurodegenerative disorders: Glial cells, including microglia and astrocytes, are central to the development and progression of neurodegenerative diseases and pain disorders. These cells are not only pivotal in normal brain function but also in the pathogenesis of various central nervous system (CNS) disorders [29].
Influence of gut microbiota on glial cells
- Regulation of glial maturation and function: Recent studies highlight the significant role of gut microbiota in influencing glial cell maturation, morphology, and function. This is largely mediated by microbial metabolites such as short-chain fatty acids (SCFAs).
- Microbial composition and glial activation: Changes in the gut microbiota composition have been observed in CNS disorders characterized by the activation of glial cells [8].
Developmental and maintenance roles of mEGCs
This provides insights into the role of microglia-like enteric glial cells (mEGCs):
- mEGCs colonize the intestinal mucosa postnatally and are continually replenished by new cells originating from the gut wall's plexi.
- Both the initial colonization and ongoing replenishment of glial cells in the intestinal mucosa are regulated by the gut microbiota [26,30].
Transcriptomic changes in glial cells
- Region-specific changes: Microglial cells in different brain regions exhibit transcriptomic changes influenced by the gut microbiome [31]. These changes are particularly pronounced in neuroinflammatory and complement system signaling pathways in the hippocampus (Hip), and RhoGDI and IL-8 signaling pathways in the prefrontal cortex (PFC).
- Impact on disease: The genes in microglia that are rescued by microbial colonization are associated with conditions like Alzheimer's disease (AD) and major depressive disorder (MDD) [32].
Behavioral and cognitive implications
Behavioral tests have shown a significant relationship between the gut microbiome and alterations in short-term memory, further supporting the observation that microglial transcriptomic changes are closely linked with neurodegenerative diseases like AD and MDD.
Dysbiosis and glial function
Microbiota dysbiosis, which can be influenced by factors such as diet, lifestyle, and genetics, contributes to altered gut permeability [6]. This, in turn, leads to protein dyshomeostasis, activation of astroglia, neuroinflammation, and cognitive decline, underscoring the critical role of gut health in brain function.
Therapeutic potential
The microbiota-gut-brain axis serves as a crucial regulator of glial functions and presents a viable target for therapeutic interventions aimed at mitigating the development and progression of neurodegenerative diseases. Potential therapeutic targets include the intestinal barrier, blood-brain barrier, meninges, and peripheral immune system [33].
Metabolite-centric mechanisms in neurodegeneration
Short-Chain Fatty Acids (SCFAs): SCFAs, primarily produced by the fermentation of non-digestible carbohydrates by gut bacteria, play a crucial role in maintaining the integrity of the intestinal barrier. They regulate the inflammatory response and enhance mucus production, which is vital for protecting the gastrointestinal tract and modulating systemic immune responses [34,35].
Aromatic amino acids and neurotransmission: Serotonin, an aromatic amino acid derived from tryptophan, activates several receptors on enteric neurons and immune cells [32]. This neurotransmitter is essential for regulating mood and gastrointestinal functions, illustrating a direct link between diet, gut microbiota, and brain function.
Tryptophan metabolites and amyloid-beta degradation: Tryptophan metabolites influence the central nervous system by modulating metalloproteinases [16]. These enzymes control the degradation of amyloid-beta peptides, which are implicated in Alzheimer’s disease, through the aryl hydrocarbon receptor (AhR) pathway.
Trimethylamine N-Oxide (TMAO) and cerebrovascular health: TMAO is produced by gut bacteria from dietary nutrients and has been detected in Cerebrospinal Fluid (CSF). It is associated with cerebrovascular diseases such as atherosclerosis and is linked to changes in synaptic receptor expression in Alzheimer’s disease models [36].
Urolithin A and neuroprotection: Urolithin A, derived from ellagic acid by gut microbiota, exhibits antioxidant and anti-inflammatory properties [37]. In animal models of Alzheimer’s disease, it has been shown to improve cognitive functions, inhibit neural apoptosis, and promote neurogenesis while reducing pro-inflammatory cytokines.
Anthocyanins and neuroinflammation: Anthocyanins are another group of microbial-derived metabolites with potent antioxidant properties. They play a role in reducing neuroinflammation and modulating cellular signaling pathways, which are crucial for maintaining neuronal health and preventing cognitive decline [4,38].
Equols and microglial protection: Equols, produced from isoflavones by gut microbiota, provide significant neuroprotective effects. They protect microglia from oxidative stress, prevent neural apoptosis, and promote neural regeneration, showcasing their potential in neurodegenerative disease management [39].
Imidazole propionate and cognitive functions: Imidazole propionate, a microbial metabolite, disrupts insulin signaling which is a critical pathway in metabolic and cognitive health [40]. This disruption is associated with the development of type II diabetes and can negatively impact cognitive functions, linking gut microbial activity with metabolic and brain health [37,41].
Sphingolipid metabolites and alzheimer’s disease: Metabolites such as dihydrosphingosine and phytosphingosine have been shown to alleviate symptoms of Alzheimer’s disease. These compounds, along with inosine and hypoxanthine, contribute to the complex interplay between gut microbiota and the central nervous system, offering potential targets for therapeutic intervention [42,34].
Early microbiome changes in neurodegenerative diseases: Detecting early changes in the microbiome of patients with preclinical Alzheimer’s and prodromal Parkinson’s disease provides insights into the temporal dynamics of microbiota alterations before overt neurodegeneration occurs [43]. This early detection is crucial for understanding disease progression and developing preventive strategies.
Microbial metabolites and microglial activation
Compelling evidence from animal studies suggests that altered gut microbiomes drive the pathogenesis of neurodegenerative diseases by modulating microglial functions and activation. These findings emphasize the importance of the gut-brain axis in neurodegeneration and the potential of microbial metabolites as therapeutic targets [44,45].
Developing targeted therapies
Identifying key microbial metabolites and understanding their precise mechanisms of action on brain function is essential for developing targeted therapeutic strategies. This approach aims to mitigate the effects of neurodegenerative diseases and improve overall brain health [46].
Targeting the intestinal and blood-brain barriers
Overview of the intestinal and blood-brain barriers: The intestinal and blood-brain barriers play critical roles in maintaining homeostasis within the body [41]. The intestinal barrier, comprising physical, chemical, and immunological components, regulates nutrient absorption and prevents harmful substances from entering the body. Conversely, the Blood-Brain Barrier (BBB) is a specialized neurovascular unit that protects the brain by preventing the passage of toxins and pathogens from the blood. Both barriers are composed of specialized cells and tight junctions that regulate substance transport [22,47,48].
Dysfunctions and their implications: Dysfunction in these barriers has been linked to several neurological disorders. For instance, compromised integrity of the BBB and intestinal barrier has been implicated in diseases such as Alzheimer’s, Parkinson’s, and multiple sclerosis [49,50]. This dysfunction allows harmful substances to bypass these protective barriers, potentially leading to inflammation and neurodegeneration [50].
Therapeutic targeting strategies
1. Enhancing barrier integrity: Various strategies have been explored to enhance the integrity of these barriers. For the BBB, methods such as passive transcytosis, intranasal administration, and stimuli-triggered disruption have shown promise. Similarly, for the intestinal barrier, the use of probiotics, prebiotics, and symbiotics has been effective in maintaining barrier function [3].
2. Advanced drug delivery systems: Nanoparticles represent a novel approach to drug delivery, designed to cross both the intestinal and blood-brain barriers effectively. These systems can improve the bioavailability and efficacy of drugs, while also reducing side effects and toxicity. Techniques like Focused Ultrasound (FUS) with microbubbles have also been utilized to transiently open the BBB, allowing for the safe delivery of therapeutic agents [13,23].
3. Modulating microbiota and immune responses: The gut microbiota and the immune system play significant roles in regulating both barriers. Modulating the gut microbiota through dietary changes or fecal microbiota transplantation has shown potential in restoring barrier function [34] Additionally, targeting the immune system with immunomodulatory drugs or therapies can help in managing barrier integrity and function [35].
4. Neurological regulation: The nervous system, particularly through components like the enteric nervous system (ENS) and the vagus nerve, also regulates these barriers. Techniques such as vagus nerve stimulation have been explored for their potential to influence barrier functions and offer therapeutic benefits for neurological disorders [51].
Emerging therapeutic approaches
The continuous research and understanding of these barriers have led to the development of innovative therapeutic strategies aimed at targeting and restoring the function of the intestinal and blood-brain barriers [20,52]. These approaches not only help in managing existing neurological conditions but also offer preventive strategies against the progression of neurodegenerative diseases.
Preclinical and clinical advances of therapeutic interventionsAlcohol use disorders and the gut-brain axis
AUD characteristics and cognitive impairments: Alcohol Use Disorders (AUDs) are marked by persistent or intermittent alcohol cravings and compulsive drinking that often lead to cognitive impairment and mood disorders [53].
Impact of intestinal microbiota imbalance: In patients with AUDs, an imbalance in intestinal microbiota is linked to the development of neuropsychiatric disorders. This imbalance includes an increase in Bacteroidetes and Proteobacteria and a decrease in Firmicutes [15].
Mechanisms of alcohol-induced mood disorders: Alcohol-induced imbalances in intestinal microflora are a contributing factor to mood disorders, with immune cells in the intestine secreting cytokines in response to this imbalance. These cytokines are then transported to the brain via the bloodstream, exacerbating memory deficits [35,37].
Therapeutic interventions in neurodegenerative diseases
1. Probiotics and cognitive function: Probiotics have shown potential in preclinical studies for improving cognitive function and reducing amyloid-beta levels in Alzheimer’s disease models. Clinical trials have also observed cognitive improvements in patients with Alzheimer’s and Parkinson’s disease following probiotic treatment [37].
2. Prebiotics and Fecal Microbiota Transplantation (FMT): Both prebiotics and FMT have demonstrated abilities to enhance cognitive functions and reduce amyloid-beta levels in Alzheimer’s disease models. A small clinical trial found FMT to be safe and well-tolerated in Alzheimer’s patients, suggesting its potential as a therapeutic intervention [54].
Dietary interventions and gut health
1. Dietary supplements and CNS disorders: Clinical and preclinical trials have investigated dietary supplements such as omega-3 fatty acids, phytochemicals, and various biotics (probiotics, prebiotics, symbiotics, and postbiotics) for their effects on psychiatric and neurological disorders [45]. These studies highlight the influence of dietary habits and interventions on conditions like depression, cognitive decline, and autism spectrum disorder.
2. SCFAs as therapeutic agents: Short-Chain Fatty Acids (SCFAs) have been identified as promising candidates for treating central nervous system disorders due to their role in modulating gut microbiota and influencing brain health [55].
Bridging preclinical findings and clinical applications
1. Translation from animal models to human studies: While numerous studies have been conducted in animal models, there is a need for more clinical studies to confirm whether the interactions observed in rodents are also applicable to humans. This gap highlights the challenge of translating preclinical findings into effective clinical therapies [32].
2. Modulating Gut microbiota for alzheimer’s disease: Modulating the gut microbiota through dietary changes or the use of prebiotics and probiotics is emerging as a promising approach to target cognitive decline associated with Alzheimer’s disease.
This exploration of therapeutic interventions within the context of the gut-brain axis underscores the complex interplay between diet, microbiota, and neurological health. These interventions offer potential pathways for managing and potentially mitigating symptoms associated with a range of CNS disorders [5,26].
Potential therapeutic approaches and future directions
Standardization and biomarker development: The advancement of therapeutic approaches within the gut-brain axis necessitates the standardization of methodologies and the identification of biomarkers [23]. This will enhance the reliability and comparability of human studies, focusing on specific areas such as Nonalcoholic Fatty Liver Disease (NAFLD), Alzheimer’s disease (AD), and autism among others. A rigorous approach involving extensive biological sample analyses will facilitate a deeper understanding of the microbiota-gut-brain interactions and their implications in various diseases [7,13].
Enhancing multi-disciplinary collaborations: To build a validated evidence base for the gut-brain axis, increasing knowledge sharing and fostering multi-disciplinary collaborations are essential. These efforts should be supported by continued public-private funding to drive forward innovative research and development in this field [11].
Personalized medicine and microbiome analysis: The integration of personalized medicine strategies, such as microbiome analysis, offers a promising avenue for tailoring dietary recommendations and probiotic prescriptions [34]. This approach is particularly relevant in managing conditions like Alzheimer’s disease, where gut health considerations are becoming integral to treatment strategies.
Psychobiotics and mental health: The emerging concept of psychobiotics highlights the potential of using exogenous factors to influence the microbiota, thereby exerting bacterially mediated positive effects on mental health. This approach has shown promise in improving symptoms of stress, anxiety, and depression, offering a novel pathway for managing mental health disorders [56].
Addressing microbial diversity loss: The loss of microbial diversity and the extinction of beneficial microbes, coupled with the expansion of opportunistic pathogens, represent significant challenges [57]. Addressing these issues is crucial for maintaining human health and requires targeted interventions to restore microbial balance.
Dietary interventions and inflammation: Many dietary benefits, particularly those related to the anti-inflammatory effects of microbial metabolites from dietary fiber and polyphenols, play a crucial role in modulating the microbiome and enhancing brain health. These findings underscore the importance of dietary interventions in therapeutic strategies [58].
The role of diet and lifestyle in modulating the axis
Influence of diet on gut microbiota and brain health: Diet significantly shapes the gut microbiota, which in turn influences brain function through various pathways. These include neuroendocrine, neural, and immune communication channels. Specific dietary components impact the composition and activity of the microbiota from birth, affecting lifelong health [5,10,12] Macronutrients play a crucial role in determining the microbial profiles, which can be modified both in the short and long term by dietary changes. This modulation of the gut microbiota by diet is pivotal, as it affects gut transit time, environmental conditions within the gut, and the availability of substrates for microbial growth [17].
Mechanisms of dietary impact on the gut-brain axis: Diet affects the brain through its influence on the microbiota via several mechanisms. These include the production of microbial metabolites, modulation of immune responses, and impacts on neuronal and metabolic pathways [5,17]. The brain and gut communicate through four main channels: the vagus nerve, microbial metabolites, control of inflammation by gut microbes, and hormone production by endocrine cells. This complex communication underscores the significant role of diet in maintaining brain health and managing mood disorders [8].
Nutritional strategies for enhancing gut-brain communication
- Consumption of probiotic and prebiotic foods: Including foods like yogurt, sauerkraut, and apples in the diet can enhance the gut microbiome, thereby supporting better mental health [57].
- Reduction of ultra-processed foods: Limiting intake of highly processed foods can decrease risks of depression and anxiety, promoting clearer thinking and improved mood.
- Inclusion of Omega-3 fatty acids: Foods rich in omega-3s, such as oily fish, are known to increase beneficial gut bacteria and reduce the risk of brain disorders [59].
- Fermented foods: Consuming yogurt, kefir, and similar foods introduces healthy microbes into the gut, which can alter brain activity.
- High-fiber foods: Diets high in fiber from sources like whole grains and fruits help maintain a healthy gut microbiota and can reduce stress hormones [8,59].
- Polyphenol-rich foods: Cocoa, green tea, and olive oil contain plant chemicals that boost gut microbiota health and may enhance cognitive functions.
Clinical and preclinical evidence
While animal studies have advanced the understanding of diet’s role in gut-brain communication, there remains a need for more clinical evidence to support these findings in human populations. The review of whole-dietary approaches emphasizes the potential of comprehensive dietary strategies to improve brain and mental health through microbiota-targeted interventions [5,29]. This approach could inform national healthy eating guidelines and contribute to the development of new dietary interventions.
Future directions in dietary interventions
The study of whole-dietary approaches represents a realistic path toward developing new dietary interventions that could significantly impact public health policies. By focusing on the entire diet rather than isolated nutrients, researchers can better understand the complex interactions between diet, the gut microbiota, and brain health. This holistic approach is crucial for developing evidence-based dietary recommendations that promote optimal brain function and mental well-being [60,61].
Challenges in research and clinical application
Standardization and methodological challenges
1. Need for standardization: The advancement of research in the gut-brain axis, particularly in Alzheimer’s Disease (AD), requires a standardized approach to methodologies to enhance the reliability and comparability of findings across different studies [20].
2. Technological challenges: Identifying the vast array of microbes in the gut microbiota is technologically demanding, primarily due to the immense diversity and the difficulty in culturing many intestinal microbes [62]. This identification is crucial for understanding their role in the gut-brain axis.
3. Dependence on advanced techniques: Current methods for identifying microbial compositions rely heavily on omics analysis, which requires sophisticated, high-tech equipment. This dependency can limit the accessibility of research in regions with fewer resources [63].
Unanswered questions in microbial influence
- Mechanisms of influence: There are significant gaps in understanding the specific mechanisms through which gut microbiota influence neuroinflammation, amyloid-beta aggregation, and oxidative stress in Alzheimer's Disease. Additionally, the role of individual microbial species or groups in modulating AD pathology remains unclear [57,58].
- Functional capacity metrics: Instead of focusing solely on the presence or absence of specific microbes, considering the functional capacity defined by metabolic pathways may provide a more accurate measure of microbiome health.
Potential and challenges in therapeutic development
- Reservoir for therapeutics: The gut microbiota presents a substantial potential as a source for new therapeutic opportunities. Targeting the microbiota-gut-brain axis could lead to novel treatments for various challenging diseases within the central nervous system [64].
- Complexity in therapeutic targeting: The complexity of the microbiota and its interactions within the gut-brain axis poses a significant challenge in developing effective therapeutic interventions that can precisely target relevant microbial components without adverse effects [53].
Throughout this comprehensive exploration of the microbiota-gut-brain axis, we have delved into the intricacies of how the gut microbiome influences not only our digestive health but also our neurological well-being and overall physiological function. The evidence presented underscores the profound impact of gut health on the development and progression of numerous diseases, ranging from neurodegenerative disorders to mood and cognitive impairments. By shedding light on the complex communication network between the gut microbiota and the brain, this paper has highlighted the critical role that diet and microbial composition play in maintaining this delicate balance and the potential for targeted therapeutic interventions to improve health outcomes.
As we move forward, it becomes increasingly clear that a deeper understanding of the microbiota-gut-brain axis could revolutionize our approach to treating and preventing a wide array of conditions. The implications of this research are vast, suggesting that future strategies aimed at modulating the gut microbiome may offer promising avenues for the development of personalized medicine and dietary interventions. The journey into deciphering the mysteries of the microbiota-gut-brain axis is far from over, but what is evident is its undeniable significance in shaping our health and paving the way for innovative approaches to enhance human well-being.
Although AI-generated tools were used to generate this Article, the concepts and central ideas it contains were entirely original and devised by a human writer. The AI merely assisted in the writing process, but the creative vision and intellectual property belong to the human author.
- Ullah H, Arbab S, Tian Y, Liu C, Chen Y, Li Q, et al. The gut microbiota–brain axis in neurological disorder. Front Neurosci. 2023;17:1225875. https://doi.org/10.3389%2Ffnins.2023.1225875
- The Gut-Brain axis. Available from: https://www.sciencedirect.com/book/9780323999717/the-gut-brain-axis
- Carabotti M, Scirocco A, Maselli M, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. PubMed. 2015;28(2):203–209. Available from: https://pubmed.ncbi.nlm.nih.gov/25830558
- Loh JS, Mak WQ, Tan L, Ng CX, Chan H, Yeow SH, et al. Microbiota–gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct Target Ther. 2024;9(1). Available from: https://doi.org/10.1038/s41392-024-01743-1
- Neren D, Johnson MD, Legon W, Bachour SP, Ling G, Divani AA. Vagus nerve stimulation and other neuromodulation methods for treatment of traumatic brain injury. Neurocrit Care. 2016;24:308-319. Available from: https://doi.org/10.1007/s12028-015-0203-0
- Fung TC. The microbiota-immune axis as a central mediator of gut-brain communication. Neurobiol Dis. 2020;136:104714. Available from: https://doi.org/10.1016/j.nbd.2019.104714
- Berding K, Vlckova K, Marx W, Schellekens H, Stanton C, Clarke G, et al. Diet and the microbiota-gut-brain axis: sowing the seeds of good mental health. Adv Nutr. 2021;12:1239-1285. Available from: https://doi.org/10.1093%2Fadvances%2Fnmaa181
- Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57(1):1–2. Available from: https://doi.org/10.1007/s00394-017-1445-8
- Nomura M, Nagatomo R, Doi K, Shimizu J, Baba K, Saito T, et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw Open. 2020;3(4). Available from: https://doi.org/10.1001/jamanetworkopen.2020.2895
- Maiuolo J, Gliozzi M, Musolino V, Carresi C, Scarano F, Nucera S, et al. The contribution of gut microbiota-brain axis in the development of brain disorders. Front Neurosci. 2021;15:616883. Available from: https://doi.org/10.3389%2Ffnins.2021.616883
- Suganya K, Koo B. Gut-Brain Axis: Role of gut microbiota on neurological disorders and how probiotics/prebiotics beneficially modulate microbial immune pathways to improve brain functions. Int J Mol Sci. 2020;21(20):7551. Available from: https://doi.org/10.3390%2Fijms21207551
- Krautkramer KA, Kreznar JH, Romano KA, Vivas EI, Barrettwilt GA, Rabaglia ME, et al. Diet-microbiota interactions mediate global epigenetic programming in multiple host tissues. Mol Cell. 2016;64:982. Available from: https://doi.org/10.1016/j.molcel.2016.10.025
- Bansal V, Costantini T, Ryu SY, Peterson C, Loomis W, Putnam J, et al. Stimulating the central nervous system to prevent intestinal dysfunction after traumatic brain injury. J Trauma. 2010;68:1059-1064. Available from: https://doi.org/10.1097/ta.0b013e3181d87373
- Gubert C, Kong G, Renoir T, Hannan AJ. Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol Dis. 2020;134:104621. Available from: https://doi.org/10.1016/j.nbd.2019.104621
- Pellegrini C, Antonioli L, Colucci R, Blandizzi C, Fornai M. Interplay among gut microbiota, intestinal mucosal barrier and enteric neuro-immune system: A common path to neurodegenerative diseases? Acta Neuropathol. 2018;136:345–361. Available from: https://doi.org/10.1007/s00401-018-1856-5
- Santisteban MM, Kim S, Pepine CJ, Raizada MK. Brain-gut-bone marrow axis. Circ Res. 2016;118(8):1327–1336. Available from: https://doi.org/10.1161%2FCIRCRESAHA.116.307709
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly Y, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569. Available from: https://doi.org/10.1126/science.1241165
- Liu L, Huh JR, Shah K. Microbiota and the gut-brain-axis: Implications for new therapeutic design in the CNS. EBioMedicine. 2022;77:103908. Available from: https://doi.org/10.1016/j.ebiom.2022.103908
- Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol Psychiatry. 2016;21:738–748. Available from: https://doi.org/10.1038/mp.2016.50
- Huang TT, Lai JB, Du YL, Xu Y, Ruan LM, Hu SH. Current understanding of gut microbiota in mood disorders: an update of human studies. Front Genet. 2019;10:1–12. Available from: https://doi.org/10.3389%2Ffgene.2019.00098
- Mayer EA. Gut feelings: The emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453–466. Available from: https://doi.org/10.1038/nrn3071
- Bhattarai Y. Microbiota-gut-brain axis: interaction of gut microbes and their metabolites with host epithelial barriers. Neurogastroenterol Motil. 2018;30. Available from: https://doi.org/10.1111/nmo.13366
- Chakrabarti A, Geurts L, Hoyles L, Iozzo P, Kraneveld AD, La Fata G, et al. The microbiota-gut-brain axis: pathways to better brain health. Perspectives on what we know, what we need to investigate, and how to put knowledge into practice. Cell Mol Life Sci. 2022;79(2). Available from: https://doi.org/10.1007/s00018-021-04060-w
- Appleton J. The gut-brain axis: influence of microbiota on mood and mental health. Integr Med (Encinitas, Calif). 2018;17(4):28–32. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6469458/
- Sandhu KV, Sherwin E, Schellekens H, Stanton C, Dinan TG, Cryan JF. Feeding the microbiota–gut–brain axis: diet, microbiome, and neuropsychiatry. Transl Res J Lab Clin Med. 2017;179:223–244. Available from: https://doi.org/10.1016/j.trsl.2016.10.002
- Palacios-García I, Parada FJ. Measuring the brain-gut axis in psychological sciences: a necessary challenge. Front Integr Neurosci. 2019;13. Available from: https://doi.org/10.3389%2Ffnint.2019.00073
- Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013. Available from: https://doi.org/10.1152/physrev.00018.2018
- Mohajeri MH, La Fata G, Steinert RE, Weber P. Relationship between the gut microbiome and brain function. Nutr Rev. 2018;76(7):481–496. Available from: https://doi.org/10.1093/nutrit/nuy009
- Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. 2019;102:13–23. Available from: https://doi.org/10.1016/j.neubiorev.2019.03.023
- Osadchiy V, Martin CR, Mayer EA. The gut-brain axis and the microbiome: mechanisms and clinical implications. Clin Gastroenterol Hepatol. 2019;17(2):322–332. Available from: https://doi.org/10.1016/j.cgh.2018.10.002
- Shortt C, Hasselwander O, Meynier A, Nauta A, Fernández EN, Putz P, et al. Systematic review of the effects of the intestinal microbiota on selected nutrients and non-nutrients. Eur J Nutr. 2018;57(1):25–49. Available from: https://doi.org/10.1007/s00394-017-1546-4
- Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8(1):42. Available from: https://genomemedicine.biomedcentral.com/articles/10.1186/s13073-016-0303-2
- Osadchiy V, Martin CR, Mayer EA. The gut-brain axis and the microbiome: mechanisms and clinical implications. Clin Gastroenterol Hepatol. 2019;17(2):322–332. Available from: https://doi.org/10.1016/j.cgh.2018.10.002
- Bui E, Fava M. From depression to anxiety, and back. Acta Psychiatr Neurol Scand. 2017;136:341–342. Available from: https://doi.org/10.1111/acps.12801
- Chen Y, Xu J, Chen Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients. 2021;13:2099. Available from: https://doi.org/10.3390%2Fnu13062099
- Jin L, Shi X, Yang J, et al. Gut microbes in cardiovascular diseases and their potential therapeutic applications. Protein Cell. 2021;12(5):346–359. Available from: https://doi.org/10.1007%2Fs13238-020-00785-9
- Abdel-Haq R, Schlachetzki JCM, Glass CK, Mazmanian SK. Microbiome microglia connections via the gut–brain axis. J Exp Med. 2019;216:41–59. Available from: https://doi.org/10.1084%2Fjem.20180794
- Bhattarai Y, Si J, Pu M, Ross OA, McLean PJ, Till L, et al. Role of gut microbiota in regulating gastrointestinal dysfunction and motor symptoms in a mouse model of Parkinson’s disease. Gut Microbes. 2021;13:1866974. Available from: https://doi.org/10.1080%2F19490976.2020.1866974
- Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol. 2020;11:25. Available from: https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2020.00025/full
- Aizawa E, Tsuji H, Asahara T, Takahashi T, Teraishi T, Yoshida S, et al. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J Affect Disord. 2016;202:254–257. Available from: https://doi.org/10.1016/j.jad.2016.05.038
- Baj A, Moro E, Bistoletti M, Orlandi V, Crema F, Giaroni C. Glutamatergic signaling along the microbiota-gut-brain axis. Int J Mol Sci. 2019;20:1482. Available from: https://doi.org/10.3390/ijms20061482
- Akbari E, Asemi Z, Daneshvar Kakhaki R, Bahmani F, Kouchaki E, Tamtaji OR, et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer's disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. 2016;8:256. Available from: https://doi.org/10.3389%2Ffnagi.2016.00256
- Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. Available from: https://doi.org/10.1038/nature11319
- Alvarez E, Martinez MD, Roncero I, Chowen JA, Garcia-Cuartero B, Gispert JD, et al. The expression of GLP1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J Neurochem. 2005;92:798–806. Available from: https://doi.org/10.1111/j.1471-4159.2004.02914.x
- Askarova S, Umbayev B, Masoud AR, Kaiyrlykyzy A, Safarova Y, Tsoy A, et al. The links between the gut microbiome, aging, modern lifestyle, and Alzheimer's disease. Front Cell Infect Microbiol. 2020;10:104. Available from: https://doi.org/10.3389/fcimb.2020.00104
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. The intestinal microbiota affects central levels of brain-derived neurotrophic factors and behavior in mice. Gastroenterology. 2011;141, 599–609.e3. Available from: https://doi.org/10.1053/j.gastro.2011.04.052
- Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. J Gastrointestinal Motility. 2011;23:1132–1139. Available from: https://doi.org/10.1111/j.1365-2982.2011.01796.x
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. PNAS. 2011;108:16050–16055. Available from: https://doi.org/10.1073/pnas.1102999108
- Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139. Available from: https://doi.org/10.1053/j.gastro.2010.06.063
- Braniste V, al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. Available from: https://doi.org/10.1126%2Fscitranslmed.3009759
- Burberry A, Wells MF, Limone F, Couto A, Smith KS, Keaney J, et al. C9orf72 suppresses systemic and neural inflammation induced by gut bacteria. Nature. 2020;582:89–94. Available from: https://doi.org/10.1038/s41586-020-2288-7
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatr. 2013;18:666–673. Available from: https://doi.org/10.1038/mp.2012.77
- Wang X, Chen Z, Geng B, Cai J. The bidirectional signal communication of microbiota-gut-brain axis in hypertension. Int J Hypertens. 2021;2021:1–9. Available from: https://doi.org/10.1155%2F2021%2F8174789
- Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013. Available from: https://doi.org/10.1152/physrev.00018.2018
- Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461–478. Available from: https://doi.org/10.1038/s41575-019-0157-3
- De la Fuente-Nunez C, Meneguetti BT, Franco OL, Lu TK. Neuromicrobiology: how microbes influence the brain. ACS Chem Neurosci. 2018;9:141–150. Available from: https://doi.org/10.1021/acschemneuro.7b00373
- Sharma RK, Yang T, Oliveira AC, et al. Microglial cells impact gut microbiota and gut pathology in angiotensin II-induced hypertension. Circ Res. 2019;124(5):727–736. Available from: https://doi.org/10.1161/circresaha.118.313882
- De Palma G, Collins SM, Bercik P, Verdu EF. The microbiota-gut-brain axis in gastrointestinal disorders: stressed bugs, stressed brain, or both? J Physiol (London). 2014;592:2989–2997. Available from: https://doi.org/10.1113/jphysiol.2014.273995
- Zhang X, Jiang X. Effects of enteral nutrition on the barrier function of the intestinal mucosa and dopamine receptor expression in rats with traumatic brain injury. JPEN J Parenter Enteral Nutr. 2015;39:114–123. Available from: https://doi.org/10.1177/0148607113501881
- Corrêa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MA. Regulation of immune cell function by short-chain fatty acids. CTI. 2016;5. Available from: https://doi.org/10.1038%2Fcti.2016.17
- Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behavior. Nat Rev Neurosci. 2012;13:701–712. Available from: https://doi.org/10.1038/nrn3346
- Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735–742. Available from: https://doi.org/10.1038/nrmicro2876
- Takiishi T, Fenero CIM, Câmara NOS. Intestinal barrier and gut microbiota: shaping our immune responses throughout life. Tissue Barriers. 2017;5. Available from: https://doi.org/10.1080%2F21688370.2017.1373208
- Cryan JF, O'Riordan KJ, Sandhu K, Peterson V, Dinan TG. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19:179–194. doi:10.1016/S1474-4422(19)30356-4. Available from: https://doi.org/10.1016/s1474-4422(19)30356-4